What Visible Signs Reveal a Precipitation Reaction: The Science Behind the Spark in Tests
What Visible Signs Reveal a Precipitation Reaction: The Science Behind the Spark in Tests
Visibility of chemical transformations plays a pivotal role in identifying precipitation reactions, offering immediate visual cues that signal key changes in solution chemistry. These reactions, notorious for producing solid deposits—precipitates—from aqueous mixtures, reveal their presence through distinct physical indicators that enable rapid, on-site detection. From the formation of colored crystals to dramatic clouding and effervescent bubbles, these telltale signs serve as universal marks of success in tests ranging from environmental analysis to clinical diagnostics.
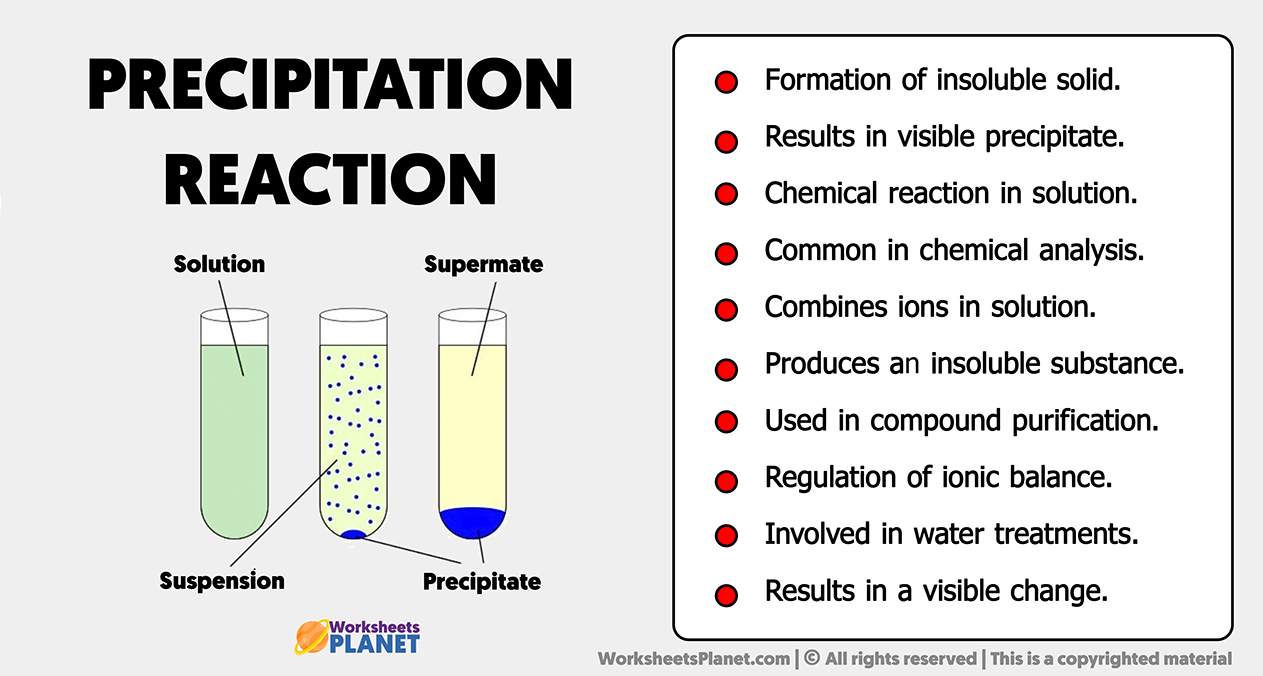
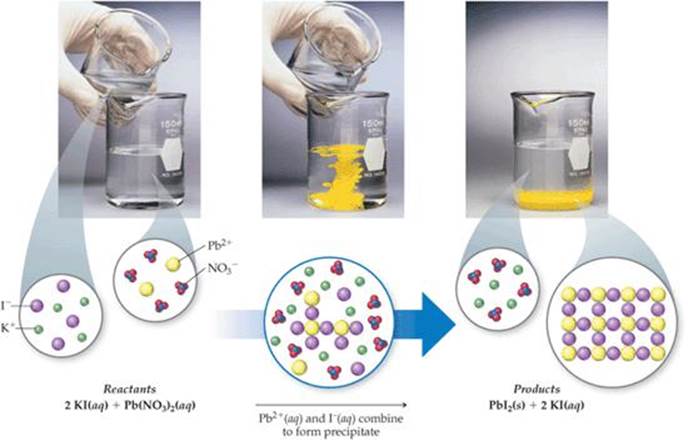
In chemistry, a precipitation reaction occurs when two soluble salts combine in water to form an insoluble compound that separates from the liquid phase as a solid—commonly known as a precipitate. But beyond the science, detectable visual hallmarks transform abstract chemical events into observable phenomena, bridging theory and real-world observation. Recognizing these signs is not merely academic—it’s essential for lab technicians, quality control specialists, and students navigating wet chemistry experiments.
The Most Reliable Visual Clues of Precipitation
Several telltale signs signal the onset or completion of a precipitation reaction. Among the most common and universally recognized are: - **Rapid Formation of a Cloudy Suspension** The emergence of a milky or opaque layer in the solution is often the first visible sign. This opacity arises as tiny insoluble particles accumulate, scattering light across the liquid.In educational settings, instructors frequently use this cloudiness to demonstrate reaction kinetics in real time. - **Visible Solid Precipitate Formation** As the reaction progresses, distinct granules, flakes, or fibers grow visibly and settle to the bottom. The texture—powdery, crystalline, or stringy—depends on the precipitate’s molecular structure.
For example, silver nitrate reacting with chloride ions yields a fine, white AgCl precipitate that easily settles and feels gritty. - **Intense Color Change Indicating Precipitate Formation** Many metal-themed precipitation reactions produce dramatic color shifts tied to insoluble salts. A classic example: mixing barium chloride with sodium sulfate produces a bright white precipitate, while silver sulfate forms whitish crystalline beads with a sharp contrast to the clear solution.
These colors not only aid observation but also aid in identifying specific ions—knowledge vital for forensic chemists and environmental analysts. - **Colloidal Cloudiness with Turbidity** Some reactions generate colloids—particles too small to settle immediately—a estado transitorio marked by persistent, diffuse cloudiness. This turbidity gradually stabilizes as particles aggregate and precipitate out, leaving clear residual liquid.
- **Effervescence or Bubbling (in Select Cases)** Though less typical in precipitation reactions, certain systems involving pH-sensitive or gas-releasing salts may bubble due to CO₂ or H₂ production. However, true precipitation-driven effervescence is rare; most observant scientists focus on optical and textural changes rather than gas evolution. - **Colorless Solution Followed by Insoluble Crystal Growth** In some cases, a clear solution gradually darkens or becomes uniformly light before the precipitate becomes fully visible.
This color gradient reflects ion concentration changes and is particularly useful in titration endpoint detection.
Sensory and Structural Markers That Confirm Reaction Completion
Beyond visual cues, several structural and sensory indicators reinforce confidence in a completed precipitation reaction: - **Crystal Morphology and Growth Rate** The shape, size, and arrangement of precipitate crystals reflect the reaction conditions. Temperature, concentration, and pH all influence crystallization dynamics.For instance, slowly cooled solutions often yield larger, purer crystals, while rapid mixing may produce amorphous or impure masses. - **Residual Solution Clarity** After stirring or settling, a solution cleared of colloidal particles signals ion complete consumption. If cloudiness lingers, it may indicate incomplete reaction or supersaturation, prompting further adjustment.
- **Tactile Feedback: Hardness and Solubility** A true precipitate feels hard and insoluble—easily scraped from glassware—unlike soluble compounds that slip between fingers. This physical property helps distinguish actual precipitation from transient cloudiness due to sampling disturbance. - **Spectral Differences** While not instantaneous, spectroscopic verification (e.g., UV-Vis or IR) often confirms precipitation by detecting shifts in absorbance linked to ion binding and solid-phase formation.
In lab practice, such tools validate what the eye observes.
Real-World Applications and Everyday Relevance
Precipitation reactions with visible signs are indispensable in multiple domains. Environmental monitoring teams rely on color-changing tests to detect heavy metals like lead or mercury in water—tiny precipitates betray even minute contamination.In medicine, diagnostic assays use precipitation to identify antibodies or antigens through visible lattice formation in immunoassays. A powerful example occurs in forensic science, where the barium sulfate test reveals sulfate contamination in unknown powders—a white precipitate instantly signaling potential hazardous material. In industrial quality control, automated optical sensors track clouding and color shifts to ensure product consistency, preventing batch failures without lab intervention.
Even in educational labs, these signs serve dual purposes: illustrating core chemistry while training attention to detail. As one laboratory instructor noted, “Students learn faster when they see—when taste, touch, and sight validate abstract concepts. A sudden cloud, a sparkling solid, a shifting hue—these are not just observations; they are proof.”
Maximizing Accuracy Through Observational Precision
Interpreting precipitation reactions demands careful attention to context.A brief haze may stem from dilution rather than reaction completion, while rapid precipitation can obscure subtleties. Best practice involves: - Recording initial solution clarity - Measuring time-to-precipitate under standardized conditions - Using contrast-enhancing slides or automated image analysis - Cross-verifying visual cues with pH or conductivity measurements - Avoiding disturbances during the settling phase These protocols ensure that what appears as a simple cloud or color shift is rigorously confirmed, turning perception into reliable data. Whether in research, industry, or education, the ability to detect a precipitation reaction through visible signs transforms chemical theory into tangible evidence.
From the delicate splash of a white crystal to the sudden darkening of a once-clear beaker, these cues are nature’s own report card—clear, immediate, and unmistakable. Understanding and interpreting them empowers scientists to act swiftly, accurately, and with confidence. In a world increasingly reliant on rapid, on-site analysis, the science of visible precipitation signs remains a cornerstone—rooting complex reactions in observable reality, one cloud at a time.

Related Post

Will Battlefield 6 Now Live on Game Pass—Battlefield’s Next Chapter Begins

Understanding The Dec 16 Zodiac: A Deep Dive Into Sagittarius Traits

Medical Insurance: Mastering Revenue Cycle Processes with Strategic Insight

Greek Trickster Unveiled: The Pandarid Role of Loki, Hermes, and Dionysus as Cultural Catalysts

