Water’s Hidden Power: How H₂O Bonding Defines Its Extraordinary Bonding and Behavior
Water’s Hidden Power: How H₂O Bonding Defines Its Extraordinary Bonding and Behavior
Water’s unique molecular architecture—governed by its distinctive H₂O bonding type—lies at the core of its life-sustaining properties. Unlike most simple molecules, water forms a dynamic, three-dimensional network of hydrogen bonds, giving rise to a cascade of physical and chemical phenomena that distinguish it from other common substances. This intricate bonding pattern not only explains its high boiling point, exceptional solvent capabilities, and the buoyancy of ice but also underpins biological processes fundamental to life on Earth.
Understanding the nature of H₂O bonding reveals why water remains indispensable across science, technology, and daily existence.
At the molecular level, each water molecule consists of two hydrogen atoms covalently bonded to one oxygen atom, adopting a bent molecular geometry. This polar covalent structure creates significant electron density differences, resulting in partial positive charges on the hydrogens and partial negative charges on the oxygen.
It is this polarity, combined with the molecule’s ability to engage in hydrogen bonding, that drives water’s unique intermolecular interactions. A hydrogen bond forms when a hydrogen atom—electrochemically attracted to the oxygen’s negative charge—interacts with a lone electron pair on an oxygen atom from a neighboring water molecule. Though individually weak (around 5–30 kJ/mol), these bonds collectively generate profound macroscopic effects, shaping water’s physical behavior in ways few substances can match.
The Molecular Basis of Hydrogen Bonding in Water
Hydrogen bonding in water arises from constitutional asymmetry: the oxygen atom in H₂O carries a negative δ⁻ charge, while each hydrogen holds a partial positive δ⁺ charge.This charge distribution enables each water molecule to act as both a hydrogen bond donor and acceptor. A typical water molecule typically forms up to four hydrogen bonds—two through its own hydrogen atoms bonded to adjacent oxygen lone pairs, and two via oxygen atoms receptively bonding with neighboring hydrogens.
This network is dynamic and constantly shifting—bond lifetimes average mere picoseconds, changing as thermal energy breaks and reforms interactions.
Despite their brevity, these transient bonds are critical to water’s fluidity and ability to mix. They allow molecules to move and rotate freely within the lattice, facilitating diffusion and transport. In contrast, substances with only van der Waals forces, like ethanol or heavier molecules, lack such robust internal connectivity.
H₂O’s hydrogen bonding thus creates a cohesive yet flexible structure, enabling efficient heat transfer and solvent action.
Macroscopic Effects of Hydrogen Bonding in Liquid Water
The cumulative strength of water’s hydrogen bond network manifests in several distinctive macroscopic properties. Consider the unusually high boiling point (100°C at atmospheric pressure) compared to similar-sized molecules like H₂S. While molecular weight is a factor, it is hydrogen bonding that primarily elevates water’s energy requirements.Similarly, water’s strong cohesive forces prevent it from vaporizing easily—a trait vital for temperature regulation in living systems and Earth’s climate. Water’s high heat capacity and thermal conductivity also stem from hydrogen bonding. Energy absorbed in raising water temperature is stored not just in kinetic molecular motion but in the energy needed to disrupt hydrogen interactions.
This capacity enables oceans to absorb vast amounts of solar heat with minimal temperature fluctuation, stabilizing global climate patterns.
Equally notable is water’s density anomaly: solid ice is less dense than liquid water due to the open hexagonal lattice formed by hydrogen bonds. This structural arrangement leaves molecules spaced farther apart than in liquid form, causing ice to float.
This seemingly minor quirk has monumental ecological consequences—floating ice insulates underlying water, preserving aquatic life during winter and regulating seasonal cycles in polar regions.
Biological and Chemical Significance of H₂O Bonding
Biological processes fundamentally depend on water’s hydrogen-bonded structure. The polar lens allows water to dissolve polar and ionic compounds, making it the universal solvent. Cells rely on aqueous environments to facilitate enzyme catalysis, nutrient transport, and cellular signaling.Unlike nonpolar solvents that disrupt biomolecular interactions, water’s ability to form transient hydrogen bonds ensures proteins, DNA, and membranes remain properly folded and functional.
Technological and Industrial Applications Rooted in Hydrogen Bonding
Beyond biology, H₂O bonding enables critical technological applications. In fuel cells and electrolysis, water’s dissociation into ions—facilitated by its hydrogen network—drives energy conversion and storage.In polymer science, hydrogen bonding governs the self-healing and mechanical strength of hydrogels and biodegradable plastics. Desalination and purification systems depend on water’s solubility and interaction dynamics, leveraging hydrogen bonds to separate impurities. Even atmospheric phenomena like cloud formation and precipitation hinge on condensation physics rooted in H₂O’s unique bonding.
“In water, hydrogen bonds are not permanent structures but a dynamic equilibrium—constantly breaking and reforming that grant the molecule remarkable adaptability and functionality,” explains Dr. Elena Torres, hydrologist and molecular physicist at Stanford Water Research Lab. “This fluid bonding network enables water to act as a solvent, insulator, temperature buffer, and structural scaffold—all essential for life and industry.” Ten key characteristics summarize H₂O bonding’s impact: - Polar covalent O-H bonds create partial charges enabling electrostatic interactions - Each molecule forms up to four hydrogen bonds, forming a flexible, dynamic lattice - High heat capacity results from energy-intensive bond disruption and reform - Unusual density trend (ice less dense than liquid) preserves aquatic ecosystems - Hydrogen bonding enables water to dissolve a vast range of solutes without breaking molecular bonds - Cohesion and adhesion forces support capillary action in plants and water transport in tissues - Thermal stability buffers
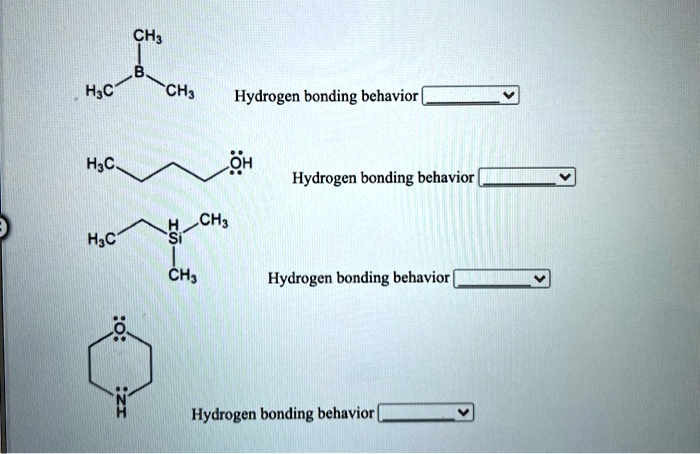
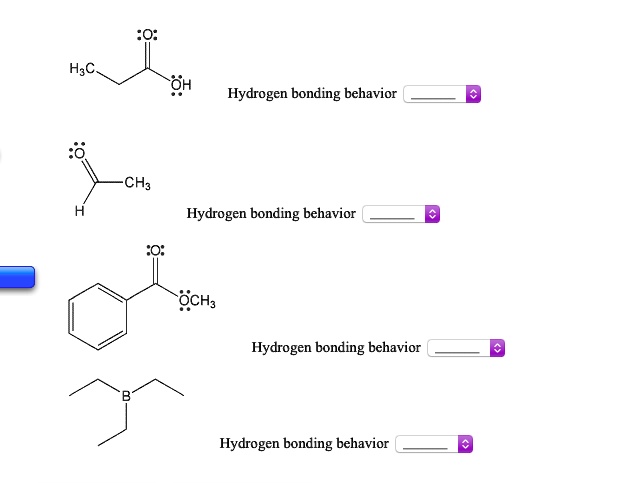
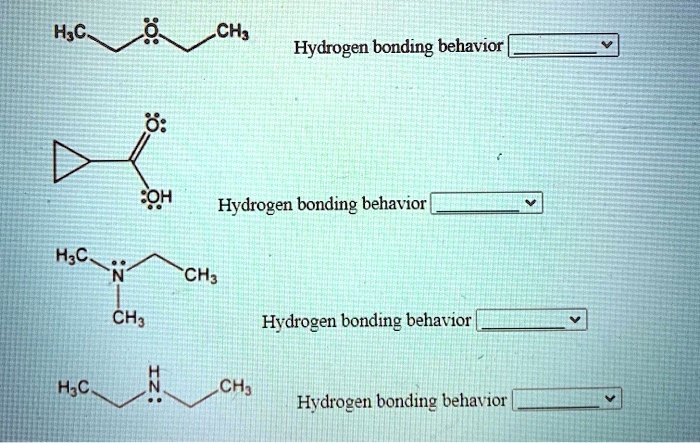
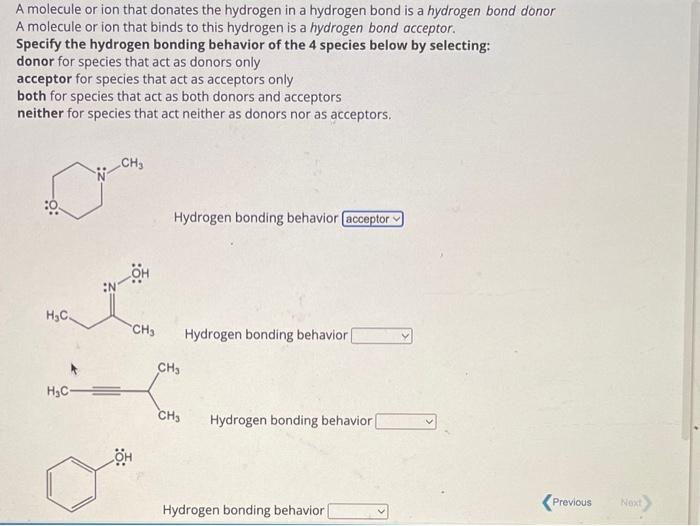
Related Post

Face-to-Face Sanctions: The Unspoken Force Shaping Behavior in Real Time
Why Extreme Picture Finders Skip or Repeat Images—Behind the Glitch and Glance
Voice Actor Of Iroh: The Timeless Soul Behind One of Animation’s Most Revered Mentors
Decoding Nyt Connections Answers Today: The Secret to Mastering Daily Word Puzzles

