Unlocking the Secrets of Ni Molar Mass: The Essential Metric That Drives Chemistry
Unlocking the Secrets of Ni Molar Mass: The Essential Metric That Drives Chemistry
In the intricate world of chemistry, few values hold as much foundational importance as the ni molar mass—though rarely mentioned outside scientific circles, this simple yet powerful metric underpins reaction calculations, molecular design, and industrial processes. Defined as the mass of one mole of a substance expressed in grams per mole, the ni molar mass is the compass scientists use to navigate stoichiometry, concentration, and purity. It ensures precision in drug development, fuel formulation, and materials science, making it indispensable in both lab and industry.
Understanding ni molar mass isn’t just academic—it is essential for anyone shaping the future of chemistry and innovation.
What Is Ni Molar Mass and Why It Defines Chemical Reactions
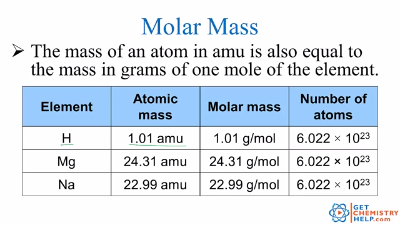
At its core, ni molar mass represents the total mass—measured in grams—associated with Avogadro’s number (6.022 × 10²³) of elementary entities, whether atoms, ions, or molecules. This value transforms abstract atomic scales into tangible, measurable quantities, enabling chemists to quantify reactants and products with precision. Each element’s ni molar mass stems directly from its atomic weight, adjusted for the mass of one mole.
For example, carbon, with an atomic weight of approximately 12.01 atomic mass units, behaves in molar form as 12.01 grams per mole. Similarly, water’s ni molar mass—18.017 grams per mole—accounts for two hydrogen atoms and one oxygen atom, encapsulating molecular identity in mass. Rotational Significance: Beyond basic mass, ni molar mass governs kinetic behavior in solution.
Molarity, defined as moles of solute per liter of solution, relies on this constant, ensuring consistent dilution and reactivity measurements. Without it, dilution formulas degrade, threatening accuracy in titrations, pharmaceutical dosing, and environmental monitoring.
The ni molar mass also serves as a gatekeeper: a liter of ethanol at STP contains about 0.789 moles, totaling roughly 14.1 grams—critical data for chemical safety and transport regulations. This ranges from blood alcohol testing to polymer production, where mass precision dictates product stability and compliance.
Calculating Ni Molar Mass: Steps, Examples, and Scientific Nuance
Determining ni molar mass follows a precise, logical sequence.
First, identify the element(s) in question and determine their atomic composition. Then, sum their individual atomic weights from the periodic table—elements like carbon, oxygen, or nitrogen are pulled directly from hard standards, while isotopic variations introduce minor but vital corrections. Consider sodium chloride: composed of sodium (Na, atomic weight ~22.99 g/mol) and chlorine (Cl, ~35.45 g/mol).
Each formula unit contains one Na and one Cl, so: Ni molar mass = 22.99 + 35.45 = 58.44 grams per mole. This compound’s molar mass becomes a factor in formulas—such as calculating grams needed for 0.1 mole reaction steps—and ensures stoichiometric balance. Ionic Compounds: Atoms Become Units: For magnesium chloride (MgCl₂), the ni molar mass integrates magnesium’s 24.31 g/mol with two chlorine atoms: 24.31 + 2×35.45 = 95.21 g/mol per formula unit. When dissolving, this value converts moles to mass, so 0.5 mole dissolves to 47.6 grams—critical for accurate industrial scaling.
Polyatomic Molecules Demand Systematic Count: Chain ethylene oxide (C₂H₄O), withNi molar mass = (2×12.01) + (4×1.008) + (16.00) = 44.05 grams per mole. This precise count informs polymerization kinetics and material property predictions, showing how ni molar mass bridges molecular architecture and function.
In bioengineering, calculating the ni molar mass of proteins—such as collagen (~Elzan ± 26,000 g/mol per molecule)—guides drug delivery and implant design, proving its role extends beyond classical chemistry into life sciences.
Applications That Shape Industry, Medicine, and Daily Life
From aspirin synthesis to clean energy, ni molar mass drives practical innovation.
In pharmaceuticals, the precise mass of active ingredients ensures consistent


Related Post

Marc Elias Wife And Family: The Quiet Power Behind A Hollywood Marriage

Law Without Borders: How Queens Volunteer Lawyers Project Transforms Access to Justice

Unlocking New Horizons: The Japan Breeding Visa for International Animal Breeders

Brittany Force Images Redefine Power: How a Visual Force Shapes Narrative and Judgment

