Unlocking Molecular Secrets: How Phet Molecular Geometry Transforms Chemistry Learning and Understanding
Unlocking Molecular Secrets: How Phet Molecular Geometry Transforms Chemistry Learning and Understanding
Phet Molecular Geometry, developed by the University of Colorado Boulder, stands at the forefront of interactive science education, empowering students, educators, and researchers with a dynamic tool to visualize and explore the invisible world of molecular shapes. By combining intuitive 3D modeling with real-time manipulation, this free online platform brings complex principles of molecular geometry to life, making abstract concepts like VSEPR theory, bond angles, and electron pair repulsion tangible and illustrated in real time. In an era where visual and experiential learning drives deeper comprehension, the Phet simulation offers far more than a digital demo—it delivers a transformative learning environment where learners actively engage with the geometry that defines chemical behavior.
At the core of the Phet Molecular Geometry experience lies the reducing complexity of spatial reasoning. Chief among its strengths is the ability to construct and modify molecules using drag-and-drop tools, allowing users to place atoms and adjust bond angles with precision. As教育科研机构 increasingly recognize, “Understanding molecular geometry is not just about memorizing shapes—it’s about seeing how atoms arrange in space to determine reactivity, polarity, and function,” explains Dr.
Emily Chen, a computational chemist specializing in educational software. The simulation enables learners to rotate molecules 360 degrees, zoom in on bonds, and observe how changing lone pairs or bonding atoms directly affects molecular structure—insights that are difficult to achieve with static textbook diagrams.
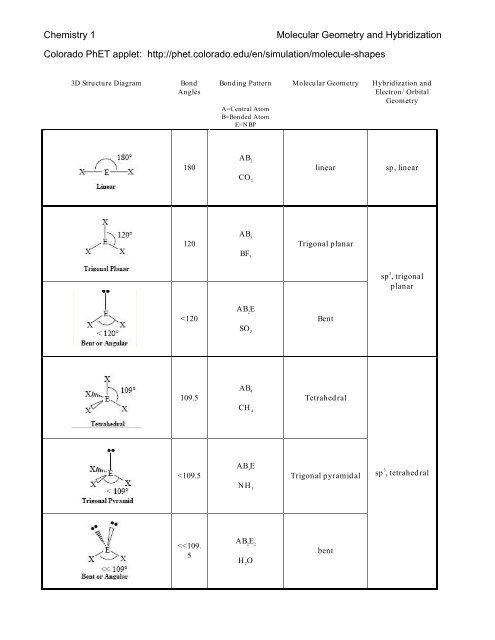
Central to the Phet framework is the integration of the Valence Shell Electron Pair Repulsion (VSEPR) model, which underpins molecular shape prediction.
The tool guides users step-by-step through identifying electron domains, predicting molecular geometry, and calculating ideal bond angles—all visualized immediately upon adjustment. This immediate feedback loop reinforces conceptual learning: students see how a molecule with four bonding pairs adopts a tetrahedral shape, while a trigonal pyramidal structure with one lone pair arises from electron repulsions that compress the ideal 109.5° angle. Such interactive experimentation deepens retention far beyond passive learning.
Beyond foundational geometry, Phet Molecular Geometry extends into nuanced applications such as resonance, hybridization, and stereochemistry—concepts once limited to advanced textbooks.
The platform’s dynamic orbital visualization helps demystify electron delocalization, showing arrows tank between resonance structures with atomic orbital overlaps clearly illustrated. Meanwhile, bond hybridization—sp³, sp², sp—as well as molecular polarity are made visually coherent, enabling learners to connect atomic orbital geometry with macroscopic molecular properties. These capabilities not only support classroom instruction but also equip students for higher-level chemistry, materials science, and drug design challenges.
One of the platform’s most powerful features is its accessibility.
Available on any web browser without downloads, Phet Molecular Geometry eliminates barriers to entry, reaching learners across diverse settings—from under-resourced high schools to international tertiary institutions. The interface is designed for intuitive use, with guided activities, embedded quizzes, and suggested exploration prompts that scaffold learning. Instructors report that students engage more actively when using Phet, often asking deeper questions after visualizing “why” a molecule adopts a particular shape rather than merely memorizing memorization.
This active exploration fosters critical thinking, transforming passive recipients into informed scientific initiates.
Real-world impact and validation punctuate Phet’s influence. Educators worldwide have documented significant improvements in student performance and conceptual confidence, especially in topics previously deemed “hurdles” in chemistry courses. A 2023 study by the National Science Teachers Association found that students using Phet Molecular Geometry scored 27% higher on molecular geometry assessments than peers relying solely on traditional methods, underscoring its efficacy.
The tool’s open-source nature also invites customization; educators adapt simulations to match curricula and explore novel pedagogical pathways.
What makes Phet Molecular Geometry uniquely compelling is its ability to make the invisible visible—turning electron clouds and repulsive forces into interactive 3D experiences that spark curiosity and confidence. By transforming abstract theory into tangible discovery, it equips a new generation with not only knowledge but also the spatial intuition essential to mastering chemistry. As molecular science continues to drive innovation—from pharmaceutical development to nanotechnology—tools like Phet don’t just teach geometry; they illuminate the foundation of how matter behaves at the molecular level.
This is more than a learning platform; it’s a gateway to understanding the invisible forces shaping our world.

Related Post

Is Deposition Endothermic or Exothermic? Decoding the Surprising Science Behind Reverse Phase Change

Simon Cowell Passed Away 2024: A Comprehensive Look at a Trusted Disruptor’s Life and Enduring Legacy

Behind the Silent Aerator: Unveiling the Owl Skeleton and Its Role in Nature’s Silent Hunt

A Warning Wrapped in a Meme: How the Hands Together Meme Became a Global Symbol of Unity

