Unlocking Cyanide’s Structure: The Explore of Its Lewis Formula and Toxic Legacy
Unlocking Cyanide’s Structure: The Explore of Its Lewis Formula and Toxic Legacy
Beneath the veil of synthetic chemistry lies a compound both vital and deadly: cyanide. Understanding its Lewis structure reveals not just atomic arrangement, but the very basis of its reactivity, toxicity, and role across industries—from metallurgy to forensic science. With a seemingly simple molecular blueprint, cyanide’s chemistry exposes deep insights into electron distribution, bonding behavior, and environmental impact.
Gr살쾕
Decoding Cyanide: The Lewis Structure That Defines Its Reactivity
At first glance, cyanide—chemical formula CnNa—appears deceptively simple, but its Lewis structure tells a more precise story. A classic representation shows a linear arrangement centered on a carbon atom bonded to a nitrogen atom, completing the molecule with azide-like character despite lacking the full ionic nature of many cyanide derivatives. The core structure for cyanide’s simplified form is written as: C≡N⁻ Where a triple bond between carbon and nitrogen dominates, supported by the resonance-stabilized anionic charge localized predominantly on nitrogen.Specific details illuminate its functionality: - The triple bond (C≡N) consists of one sigma and two pi bonds, forming one of the strongest single bonds in organic chemistry—accounting for cyanide’s resistance to thermal degradation under normal conditions. - Nitrogen carries a formal negative charge in standard Lewis models, reflecting its electronegativity and affinity for hydrogen bonding, a trait critical in biological interactions. - Carbon maintains a complete octet, though its sp-hybridized bonding influences directional reactivity in chemical reactions.
- Resonance structures, though limited, show subtle charge delocalization that influences cyanide’s role in nucleophilic substitutions and metal complex formation. This arrangement, while simplified, underpins cyanide’s dual identity: a match made in both utility and danger. Its linearity and charge distribution make it a potent reagent in analytical chemistry and industrial processes, while the fixed negative polarity enables binding to metal ions—critical in toxicity mechanisms.
From Industrial Workhorse to Toxic Menace: The Dual Nature of Cyanide
Cyanide’s utility is deeply embedded in modern chemistry. Widely deployed in metallurgical extraction—particularly in gold leaching—it facilitates the efficient conversion of metallic ores into soluble cyanide complexes, revolutionizing mining efficiency. Yet, its very chemistry fuels its lethality.The negative charge on nitrogen allows cyanide ions (CN⁻) to bind tightly to transition metals in cytochrome c oxidase, an enzyme central to cellular respiration. By inhibiting electron transfer, cyanide disrupts ATP production, leading to rapid cellular asphyxiation—a process described by chemist Vera Eckstein as “the silent sabotage at the heart of mitochondria.” Regulatory thresholds emphasize the compound’s potency: concentrations as low as 0.01 mg/L in water can prove fatal, underscoring its extreme hazard. Forensic toxicology often centers on detecting cyanide derivatives such as hydrogen cyanide (HCN), the volatile and airborne form frequently implicated in acute poisoning cases.
Structural Insights and the Path Forward
The Lewis structure of cyanide—though deceptively minimal—serves as a gateway to understanding its broader implications. Its triple-bonded, charge-distributed geometry informs both synthetic applications and risk assessment, guiding safer handling protocols and remediation strategies. For engineers and toxicologists alike, mastering this structure is essential: it transforms abstract bonding into actionable knowledge.Emerging research continues to probe cyanide’s behavior at molecular interfaces, seeking ways to neutralize its toxicity or repurpose its reactivity in controlled environments. Advances in computational chemistry now model cyanide-metal interactions with precision, supporting both industrial optimization and medical intervention. In the end, the cyanide molecule—through its elegant Lewis blueprint—embodies a paradox: a cornerstone of progress shadowed by profound danger.
Mastery of its structure ensures that humanity advances responsibly, wielding knowledge not just to innovate, but to contain.

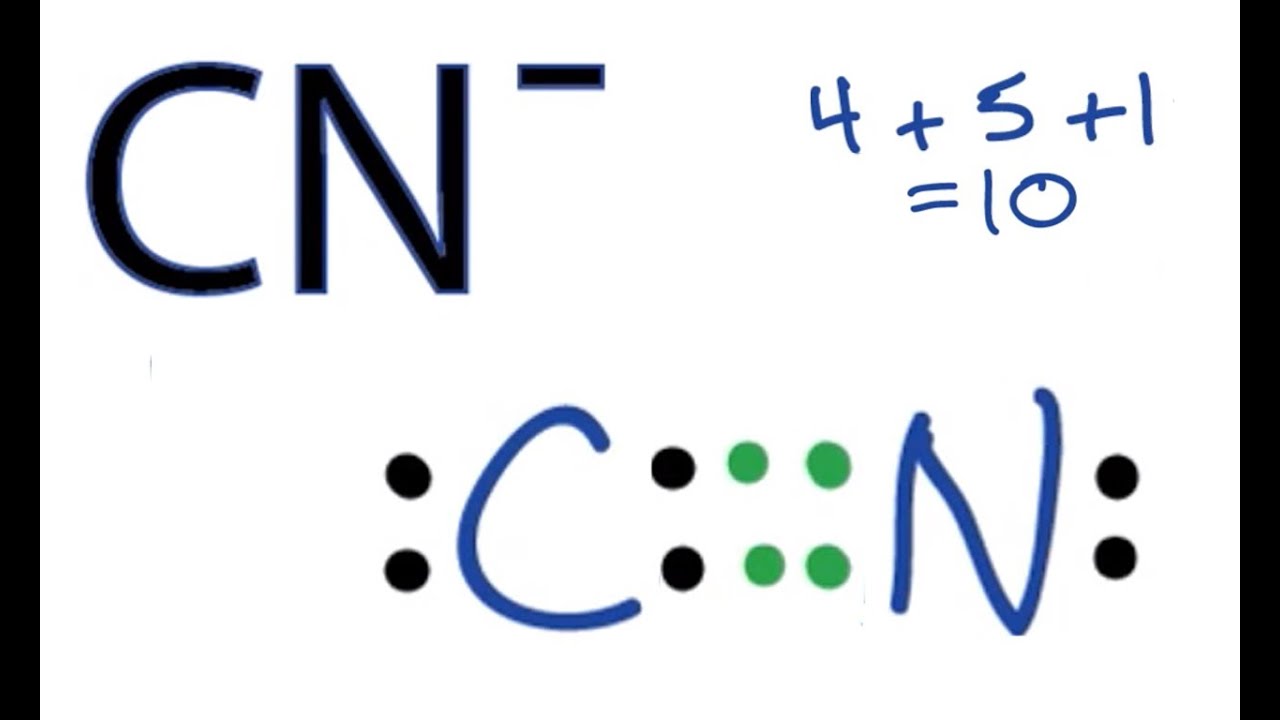


Related Post
Standard Margin Size for Printing: The Shrinking or Steady Square That Shapes Professional Documents

Missouri State Football Stadium: Heartbeat of Southern Missouri Athletics

Jackson Hole Tours: Where Wild America Meets Curated Adventure

The Unifier of Emotion and Identity: Rosabell Laurenti’s Vision in Contemporary Art

