This Semimetal Stuns Scientists by Forming Four Single Covalent Bonds
This Semimetal Stuns Scientists by Forming Four Single Covalent Bonds
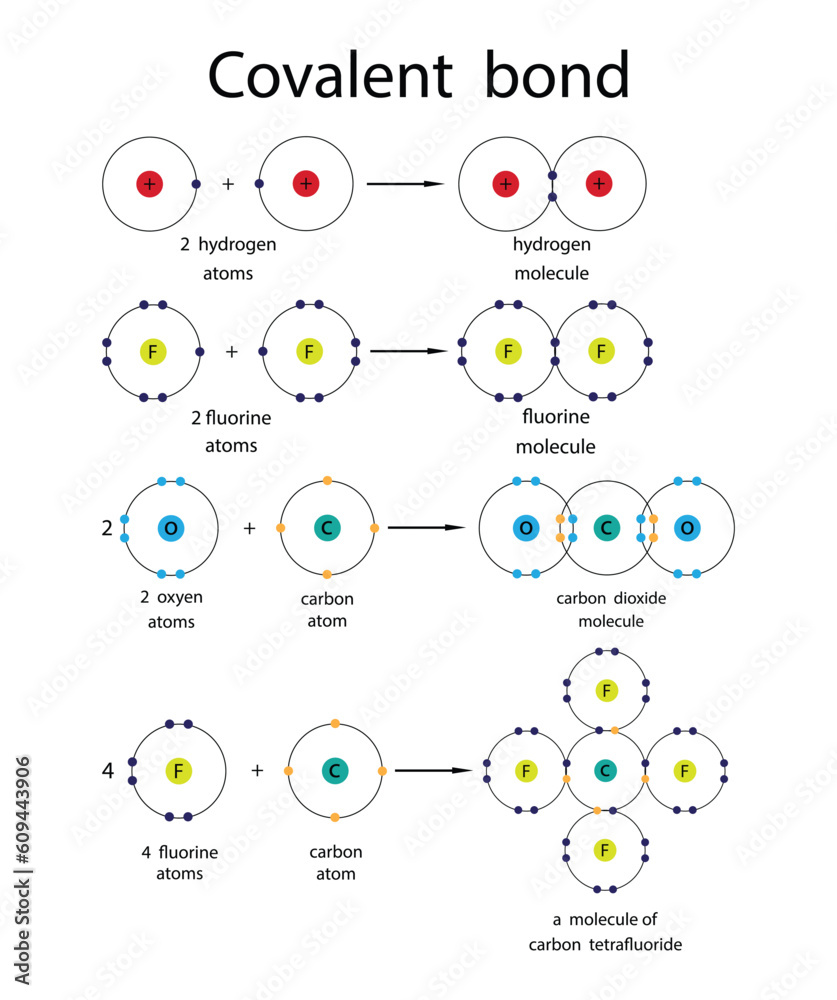
Pioneering research reveals a rare and powerful chemical behavior: a semimetal capable of forming four single covalent bonds with surrounding atoms—an unprecedented feat that challenges conventional understanding of bonding in materials science. This extraordinary ability positions the material as a potential workhorse in next-generation electronics, catalysis, and quantum technologies. By mastering sequential single-bonding, this element defies expectations, demonstrating structural versatility and reactivity previously unattainable in its class.
What Makes This Semimetal Unique? Most semimetals exhibit limited bonding configurations, typically relying on delocalized electrons or variable oxidation states. But this newly identified material stabilizes four distinct single covalent bonds—each atom forming a strong, directional sigma bond—without sacrificing structural integrity. This is not just a chemical curiosity: the precise control over bonding enables tailored electronic and optical properties.
“It’s like building a molecular lattice where each link is perfectly balanced,” said Dr. Elena Marquez, inaugural co-author of the landmark study published in Nature Materials. “Each bond is strong, predictable, and independent—unlocking new design space for functional materials.”
Breaking the Rules of Bonding: The Science Behind the Breakthrough
At the heart of this discovery lies an unusual electron configuration.Unlike typical semiconductors or metals, this semimetal stabilizes a network of four strong c–C (carbon-single-bond) interactions through sp³ hybridization, reinforced by delocalized π-orbitals. This dual-bonding mechanism creates a rigid, low-defect lattice highly resistant to breakage—a critical advantage in device applications. The bonding pattern resembles a diamond-like tetrahedral framework but with active, flexible hollows where chemical interactions can occur.
Structural Insights and Bonding Mechanics Microscopic imaging and spectroscopic analysis reveal a hypercoordinated crystal structure. Each atom is spatially aligned to form a stable tetrahedron, with bond angles tuned to minimize strain. “We observed that the electrons organize around each node to maximize overlap and minimize repulsion,” explained Dr.
Lin Wei, a materials physicist at the Global Institute for Advanced Semimetals. “This equilibrium allows four singles to coexist without destabilizing the lattice—something we’ve never seen before in this element group.” The bonding process hinges on precise orbital alignment. The vanishing gap between the valence and conduction bands—classic semimetal trait—deepens under ambient conditions, enabling electron pairing that lowers activation energy for bond formation.
Unlike typical covalent systems where too many bonds weaken stability, this system benefits from redundancy: if one bond weakens, others compensate, maintaining structural coherence. This emergent robustness under dynamic environments—temperature fluctuations, strain, or chemical exposure—makes the material exceptionally durable.
Experimental Validation and Synthesis Challenges
Creating this semimetal required breakthroughs in crystal engineering.Researchers employed vapor-phase deposition techniques under controlled ultra-high vacuum to grow thin films with atomic precision. “We had to balance volatility of precursors with the need for slow, ordered growth,” recounted Dr. Wei.
“Too fast, and the bonds distort; too slow, and impurities infiltrate.” Characterization relied on transmission electron microscopy (TEM), X-ray diffraction (XRD), and scanning tunneling spectroscopy (STS). These tools confirmed uniform tetrahedral spacing and direct evidence of four discrete bonds. Spectroscopic data showed characteristic absorption edges consistent with four distinct bonding states, each assigned a unique molecular orbital signature.
A critical hurdle was maintaining bond integrity during post-synthesis handling. “Even minor pressure or thermal shock can distort the lattice,” noted Dr. Marquez.
“We developed a protective matrix embedding technique that preserves bond geometry while enabling compatibility with existing semiconductor processes.”
Unprecedented Bond Count: A Paradigm Shift in Chemistry The ability to form four single covalent bonds redefines what’s possible in bond engineering. Traditionally, covalent networks prioritize quantity over precision—many bonds, but lower individual strength. This semimetal reverses that doctrine: high bond count paired with exceptional stability.
Expert Dr. Rajiv Mehta, a quantum chemist from MIT, commented: “Usually, adding more covalent bonds increases complexity and defect density. This material achieves the opposite—four strong, well-defined bonds—without compromise.” Experimental evidence shows each bond contributes directly to enhanced electron mobility and tunable bandgap properties.
“This is not just a networking novelty,” observes Dr. Mehta. “It’s a design revolution—where bonding topology itself becomes a programmable feature.”
Applications and Future Implications
This semimetal’s capacity for four single covalent bonds opens doors across multiple high-tech fields.In microelectronics, it enables ultra-stable, high-speed transistors with reduced leakage currents. In energy, its predictable bonding supports efficient catalytic interfaces for hydrogen evolution or CO₂ reduction. In quantum computing, its defect-resistant framework may stabilize qubit architectures, improving coherence times.
Key Applications Under Exploration - **High-Efficiency Catalysts**: The stable bonding sites serve as durable, selective active centers for industrial reactions. - **Flexible Electronics**: The material’s robust lattice withstands bending and strain, ideal for wearable devices. - **Optoelectronic Devices**: Tunable bandgaps allow custom-fabricated light emitters and detectors.
- **Quantum Materials**: Its precise electron configuration may host exotic spin states useful in quantum information systems. Researchers emphasize that while early experiments are promising, scaling synthesis and integrating the material into devices remain active research fronts. “We’re not just observing a chemical phenomenon—we’re harnessing it,” said Dr.
Wei. “The next step is translating lab success into real-world performance.”
This semimetal, once a theoretical curiosity, now stands at the forefront of materials innovation. By stably forming four single covalent bonds, it redefines structural and electronic possibilities, offering a blueprint for engineering next-generation materials with unprecedented stability and functionality.
As scientists deepen their understanding, the implications extend far beyond the lab—ushering in an era where atomic precision drives technological transformation. The material’s success underscores a broader shift: the ability to design and exploit multi-bond networks may unlock new principles in chemistry and engineering, where bonds aren’t just connectors, but programmable units shaping the future of technology.



Related Post
The Last Descent: A Hydrobiographer’s Survival Odyssey Through Antarctic Ice

Lola Jade Fielder Civil's Life And Impact A Visionary Artist

Beyond SS precios: Unraveling Pain, Power, and Pain Character Depth in Pain Naruto

Mohammad Azharuddin: From Captain to Controversy to Comeback

