The Surprising Science Behind Physical Changes: How Matter Transforms Without Losing It
The Surprising Science Behind Physical Changes: How Matter Transforms Without Losing It
From melting ice cubes to rusting metals, physical changes shape the world around us every moment — yet remain fundamentally different from the dramatic transformations of chemical reactions. Unlike chemical changes, which forge entirely new substances by altering molecular bonds, physical changes preserve a material’s identity while reshaping its form through heat, pressure, or phase transitions. The quiet power of physical change lies in its ability to alter appearance, state, or structure without rewriting molecular blueprints—a dynamic that influences everything from daily life to industrial innovation.
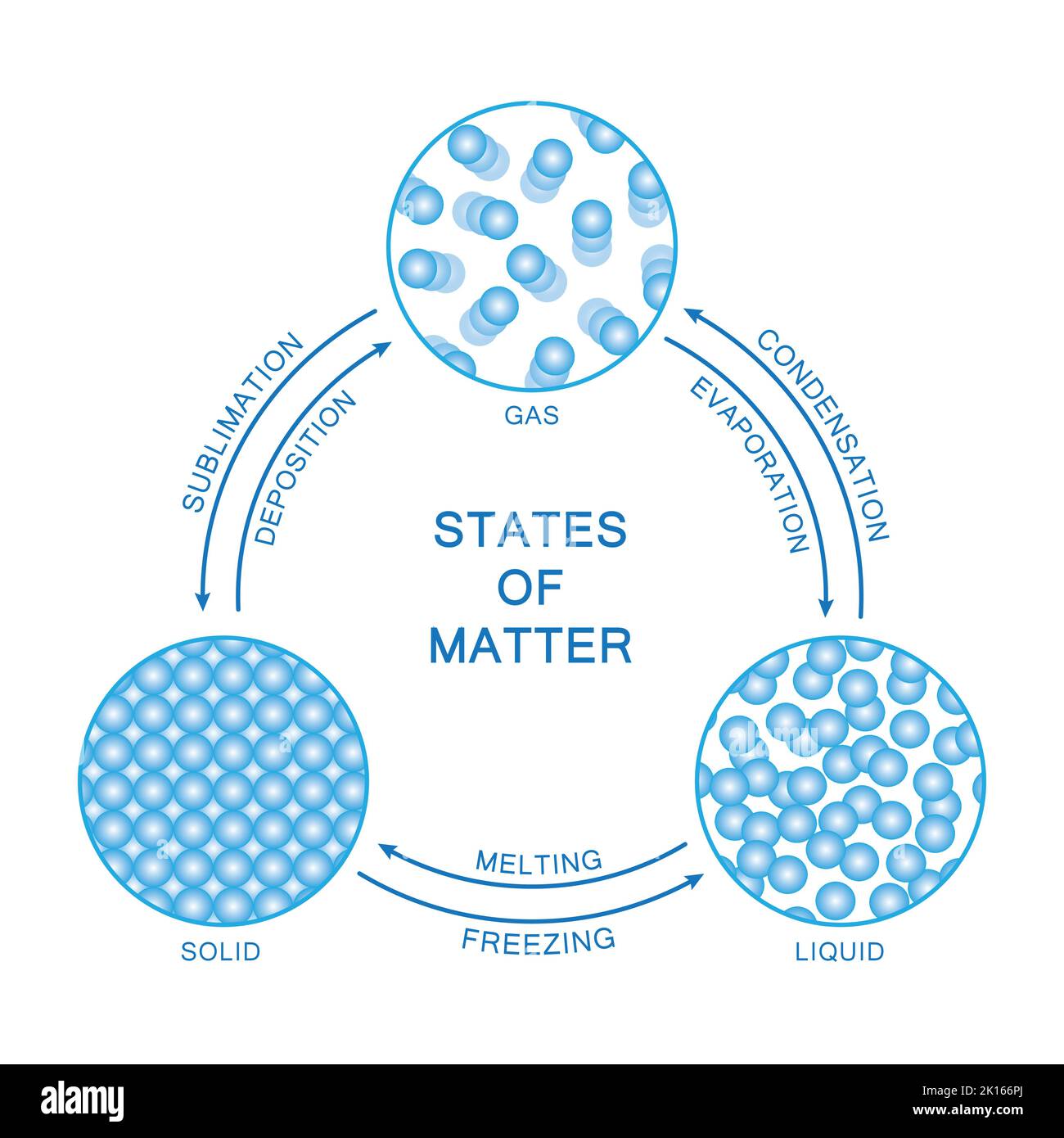
Understanding physical change means recognizing key processes like melting, freezing, condensation, evaporation, and sublimation—not as mere alterations, but as essential transformations governed by precise scientific principles. These processes involve energy exchange, most often heat, yet do not create new chemical entities. “Physical change is when matter changes form, not function,” explains Dr.
Elena Marquez, materials scientist at the Institute for Applied Physics. “The atoms stay the same; only their arrangement or energy state shifts.” The main mechanisms of physical change unfold through four primary pathways, each demonstrating distinct behaviors under varying environmental conditions.
Melting: From Solid to Liquid — A Phase Transformation Without Loss
Melting occurs when a solid absorbs sufficient thermal energy to overcome its rigid lattice structure, transitioning into a liquid state.This shift is marked by temperature-specific thresholds—freezing point for water at 0°C, however, less arbitrary: ice remains chemically H₂O while becoming liquid H₂O, full of the same molecules. “What changes is order, not substance,” notes Dr. Matthew Reed, professor of physical chemistry.
“When ice melts, water molecules gain mobility but retain H₂O bonds.” Factors influencing melting include impurities (which lower the melting point, a principle exploited in ice sculpting and road de-icing), pressure, and external heat sources. The process is fully reversible: when liquid water cools, crystallization restores structure without altering chemistry.
Freezing: Reversing the Transformation — The Universe’s Favorite Backtracker
Freezing is the converse of melting—rate-controlled crystallization of liquid into solid as thermal energy dissipates.Unlike chemical reactions, freezing retains molecular integrity: water becomes ice without molecular breakage. This phase reversal is so predictable that refrigeration technology hinges on precise control of freezing points, using antifreeze compounds to prevent damaging ice formation in engines and biological tissues. While the physical state flips between fluid and solid, no hydrogen or oxygen atoms are rearranged.
This reliability makes freezing indispensable in food preservation, cryogenics, and climate systems, where timing and temperature dictate success.
Condensation and Evaporation: States of Water — The Cycle of Change
Two adjacent processes governing water’s behavior: evaporation removes heat, transforming liquid water into vapor (gas), while condensation reverses it—vapor absorbing energy to form droplets. These transitions define the hydrological cycle but exemplify physical change at its most visible and vital.Evaporation is active: molecules escape surface tension based on molecular speed and environmental heat. Condensation occurs when vapor cools, meeting energy thresholds to reforge intermolecular forces. “Water vapor condensing on a cold glass is a textbook example of energy exchange without chemical change,” explains Dr.
Marquez. “The water’s H₂O remains unchanged—only state and kinetic energy are altered.” These dynamics regulate weather patterns, fuel cloud formation, and drive cooling systems in human habitats, proving physical change is not passive but pivotal to Earth’s equilibrium.
Sublimation and Deposition: From Solid to Gas (and Back) Without Liquid
Less intuitive but scientifically profound are sublimation—solid turning directly into gas—and deposition, its reverse.Dry ice (solid CO₂) sublimates at atmospheric pressure, expanding into gas without thawing, a process


Related Post

New York Mets Coaching: How Leadership Shapes a Winning Identity

Find Your Reno Home by Zip Code: Precision Addressing Unlocks the Heart of Nevada’s Thriving City

Bryan Adams Married: Love, Longevity, and Legacy in a Rock & Roll Icon’s Life

Watch Wahui Result Live: Where Every Match Unfolds in Real Time

