The Boiling Point of Water in Kelvin: A Scientific Benchmark Defining Thermodynamics
The Boiling Point of Water in Kelvin: A Scientific Benchmark Defining Thermodynamics
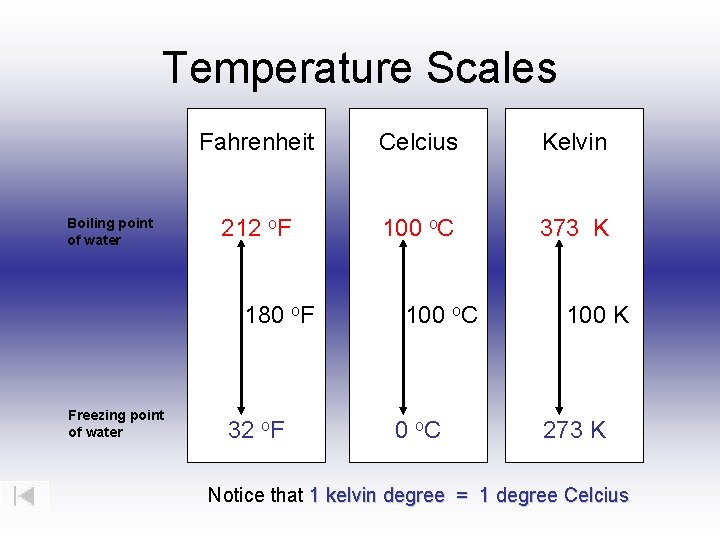
At exactly 373.15 Kelvin, water transitions from liquid to vapor—a pivotal threshold governed by the Kelvin scale, the cornerstone of absolute temperature measurement in science and engineering. This precise point, far beyond everyday experience, marks the beginning of the vaporization phase and serves as a fundamental reference in chemistry, climate science, and industrial processes. Understanding boiling point in Kelvin reveals not just a number, but a window into molecular behavior under absolute zero thresholds.
Boiling temperature is conventionally measured in degrees Celsius (°C), but scientific rigor demands consistency through standardized absolute scales. The International Temperature Scale, based on the Kelvin, defines 0 K as absolute zero—the theoretical limit where particle motion ceases. Water’s boiling point at 373.15 K sits midway between ice formation at 273.15 K and superheated vapor conditions, making it a critical benchmark in thermometry.
As Dr. Elena Petrova, thermal physicist at ETH Zurich, notes: “This 373.15 K value is not arbitrary—it emerges from systematic experimentation and statistical mechanics, ensuring repeatable and reliable measurements across global laboratories.” ### The Kelvin Scale: Foundation of Absolute Temperature The Kelvin scale, defined by the triple point of water (273.16 K), establishes 0 K as the coldest conceivable temperature, far below any natural environment. Unlike Celsius or Fahrenheit, Kelvin increments reflect real thermodynamic increments tied to energy at the molecular level.
Here, the boiling point of water in Kelvin—373.15—represents a macro-scale expression of nanoscale atomic dynamics. Since energy transfer governs phase changes, knowing this precise Kelvin value ensures accuracy in modeling heat transfer, chemical reaction kinetics, and atmospheric processes. Engineers rely on it when designing high-temperature systems, from nuclear reactors to spacecraft thermal shields.
### Thermodynamic Implications of 373.15 K At 373.15 K, water molecules possess sufficient kinetic energy to overcome intermolecular hydrogen bonds and transition into the gas phase. This energy threshold aligns with enthalpy of vaporization values measured near this point, confirming its scientific significance. In industrial applications, power plants use precise boiling temperatures to optimize steam cycle efficiency, while polymerization processes are carefully controlled at or near this Kelvin value to ensure product consistency.
As renewable energy systems advance, accurate temperature transitions at 373.15 K remain vital to improving thermal storage and solar thermal conversion technologies. ### Calibration and Measurement Standards Accurate determination of water’s boiling point at 373.15 K demands highly controlled laboratory conditions. Modern differential scanning calorimetry (DSC) and cryogenic thermometry instruments provide sub-0.01 K precision, enabling repeatable validation.
National Metrology Institutes, such as NIST in the United States and PTB in Germany, maintain realizations of the Kelvin using atomic and quantum standards. These metrological anchors ensure that laboratory results globally align with the defined boiling temperature, supporting everything from academic research to industrial certification. Without such traceability, discrepancies could undermine critical applications in climate modeling, pharmaceutical manufacturing, and semiconductor fabrication.
### Real-World Applications Beyond the Lab The boiling point of water at 373.15 K is not merely academic—it shapes technologies and natural phenomena alike. In environmental science, understanding phase transitions at this Kelvin value aids in modeling cloud formation and precipitation dynamics. In medicine, sterilization processes depend on precise vapor conditions, often requiring extended exposure at 373.15 K or above to reliably eliminate pathogens.
Meanwhile, aerospace engineers incorporate this threshold when developing thermal protection systems for re-entry vehicles, where extreme heat generation parallels vaporization physics. Even everyday devices such as pressure cookers exploit differences in boiling points across controlled environments—illustrating how deeply embedded 373.15 K is in human innovation. The exact value of 373.15 K transcends a single temperature reading; it embodies a convergence of fundamental physics, rigorous metrology, and practical engineering.
As temperature measurement evolves toward quantum precision, this Kelvin benchmark remains a linchpin—anchoring scientific consistency across disciplines and enabling breakthroughs in energy, medicine, and environmental science. In the end, the boiling point of water in Kelvin stands as more than a number. It is a testament to human ability to quantify nature’s extremes and harness them for progress—one controlled vaporization at 373.15 K, powering both discovery and application.



Related Post

Cozumel’s January Weather: A Beacon of Warmth in the Caribbean’s Shoulder Season

Understanding The Age Of Gutfeld’s Wife: A Detailed Insight into Her Identity, Relationship, and Public Presence
Time At KSA: The Pulse of Productivity in Saudi Arabia’s Evolving Work Landscape

U.S. Citizenship Cases at a Crossroads: A Deep Dive into USCIS Case Status and Reform Challenges

