The Acid-Base Puzzle: Mastering pH and pOH Answers in Everyday Science
The Acid-Base Puzzle: Mastering pH and pOH Answers in Everyday Science
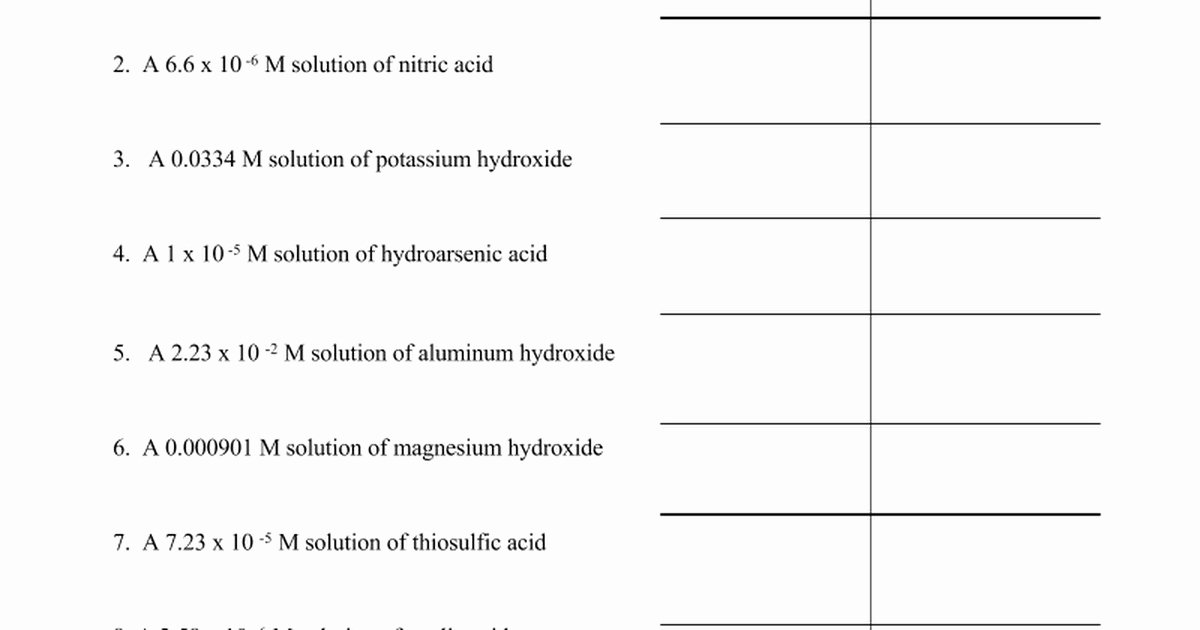
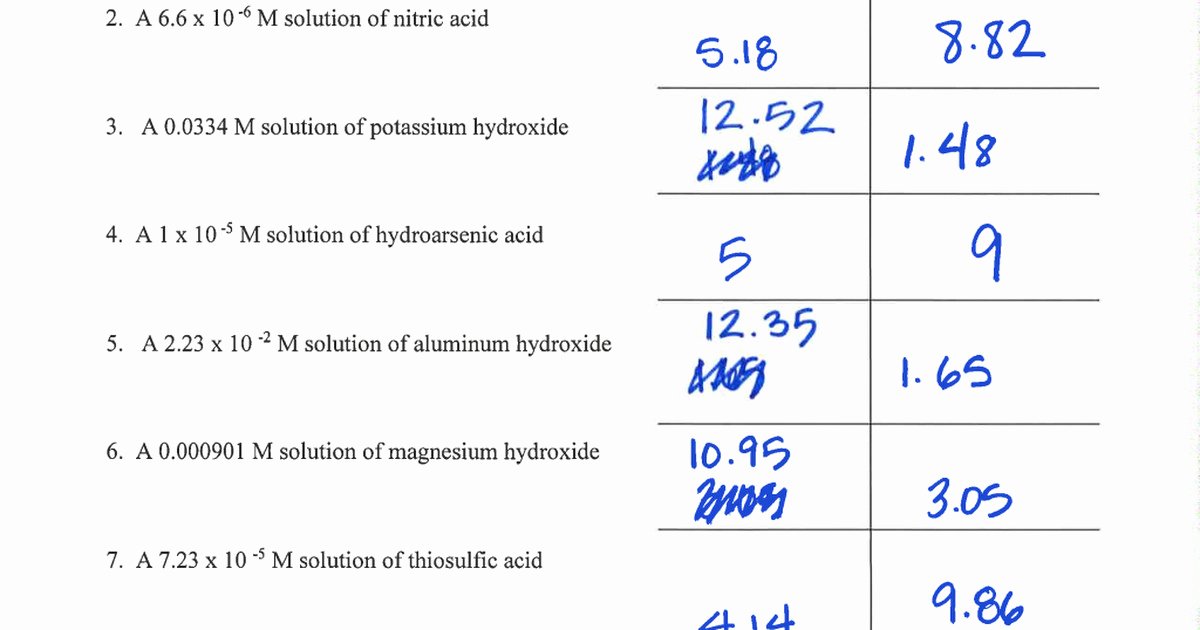
Understanding pH and pOH is fundamental to unlocking the chemistry behind countless natural and industrial processes—from soil health and water quality to pharmaceutical formulation and human physiology. The correct interpretation of these logarithmic scales determines how acids and bases behave in solution, guiding everything from lab experiments to agricultural practices. Based on Problem Set 9.2, students and professionals alike face recurring challenges in translating pH and pOH answers into meaningful, actionable insights.
This deep dive explores the core principles, problem-solving strategies, and real-world applications behind pH and pOH calculations, ensuring confidence in mastering this essential chemistry domain.
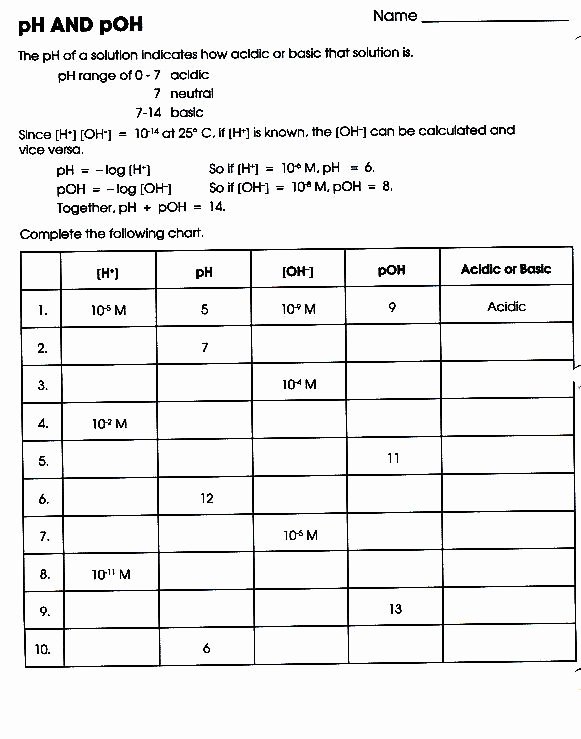
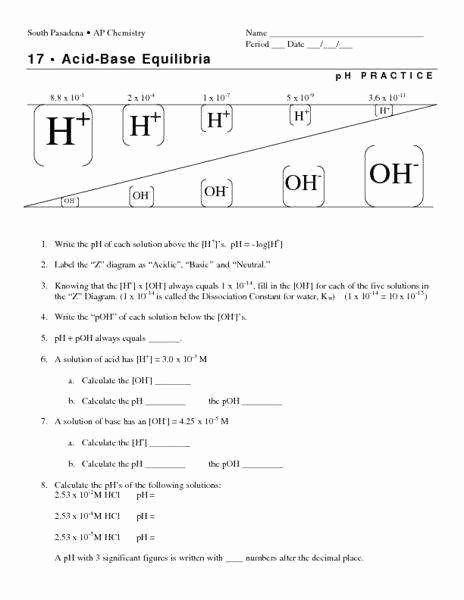
pH and pOH are logarithmic measures rooted in the concentration of hydrogen ions in aqueous solutions, defined by well-established mathematical relationships. The pH scale quantifies acidity or basicity using the formula pH = –log₁₀[H⁺], where [H⁺] represents the molar concentration of hydrogen ions in moles per liter.
Similarly, pOH is derived from hydroxide ion concentration via pOH = –log₁₀[OH⁻]. Since water autoionizes to produce equal concentrations of H⁺ and OH⁻—6.0 × 10⁻¹¹ mol/L at 25°C—the relationship pH + pOH = 14 becomes a cornerstone of acid-base equilibrium. This interdependence ensures that precise understanding of one scale enables accurate inference of the other, a critical insight in both theoretical and applied chemistry.
The Role of pKa, Buffers, and Misconceptions
Textbooks and Problem Set 9.2 reinforce that pH calculations extend beyond basic logarithms, incorporating concepts like pKa, buffer systems, and equilibrium dynamics.The pKa of a weak acid, defined as –log₁₀(Kₐ), helps predict acid dissociation strength—lower pKa values indicate stronger acids. For weak acids, the Henderson-Hasselbalch equation (pH = pKa + log([A⁻]/[HA])) becomes indispensable, linking pH directly to the ratio of conjugate base to acid concentrations. This principle underpins buffer design, essential in biochemical assays and drug delivery systems.
A frequent stumbling block highlighted in problem sets involves confusing pH with pOH. While pH captures hydrogen ion activity, pOH reflects hydroxide availability—yet both are intrinsically linked. Misinterpreting the relationship between pH and pOH often stems from neglecting temperature dependencies.
At 25°C, pH + pOH = 14, but deviations occur with extreme temperatures, altering ion product constants. “Many students mistakenly assume pOH equals pH when in reality," notes Dr. Elena Torres, physical chemist and science educator, "unless explicitly told the system is neutral.
Precision demands recognizing these interconnections.”
Step-by-Step Problem Solving: From Theory to Practical Answer
Mastering pH and pOH answers requires systematic application of core formulas and conceptual clarity. Consider a standard acid-base problem: a 0.1 M solution of acetic acid (pKa = 4.76). Applying the Henderson-Hasselbalch equation gives pH = 4.76 + log(0.1/[A⁻]), assuming weak acid dominance.But caution is vital—if the buffer capacity is exceeded, pH shifts dramatically, undermining predictions. Students must identify whether a solution is acidic, basic, or neutral and select appropriate models: strong acids/bases for complete dissociation, weak substances for equilibrium-based analysis, and buffer systems for physiological relevance. Real-world problem sets often present mixed conditions—such as adding a strong acid to a buffer—requiring deduction of new pH values through dilution, neutralization, or Le Chatelier’s principles.
For instance, adding HCl to a buffer containing acetic acid and acetate reduces acetate concentration, increasing pH slightly but keeping it stable through shift equilibrium. “The key is recognizing that buffers resist pH change, but only within limits,” explains Dr. Torres.
“Understanding this preserves accurate predictions in dynamic systems.”
Beyond lab exercises, pH and pOH play pivotal roles in agriculture, where soil pH dictates nutrient availability—most crops thrive in slightly acidic to neutral conditions (pH 6–7). In water treatment, pH monitoring ensures effective coagulation and disinfection, while in biology, enzyme function hinges on precise pH environments. These applications underscore that pH and pOH are not abstract nomenclature but practical tools shaping modern science and industry.
The Road to Confidence: Strategies for Success
Mastering Problem Set 9.2’s pH and pOH questions demands intentional practice and reinforcement of key concepts.Learners should routinely convert between pH and pOH using pH + pOH = 14, especially when working with approximations or logarithmic functions. Visualizing the ion product of water (Kw = [H⁺][OH⁻] = 1.0 × 10⁻¹⁴ at 25°C) anchors calculations in fundamental thermodynamics. Moreover, integrating addition and dilution scenarios into mental models strengthens adaptability—critical when real-world data rarely conform to textbook idealism.
Challenging misconceptions further solidifies understanding. For example, hydrochloric acid (HCl) does not “neutralize” pH instantly; its strength determines proton release speed, with concentrated solutions reacting rapidly. Similarly, pOH is not a standalone scale but a reflection of OH⁻ under specific equilibrium conditions.
“Quick confirmation questions—‘Is pH positive with acid? Is pOH only relevant in basic solutions?’—are powerful checks,” suggests Dr. Torres.
Ultimately, pH and pOH are not just formulas to memorize but lenses through which to interpret chemical behavior across disciplines. Problem Set 9.2’s exercises distill complex principles into manageable challenges, teaching learners to parse equilibrium, apply approximations, and recognize temperature and concentration nuances. Success in these problems equips professionals and students alike to tackle real consequences—from optimizing fertilizer blends to diagnosing patient acid-base balance—with precision and purpose.
The mastery of pH and pOH answers transcends academic success, forming a foundation for informed decision-making in countless fields.By unraveling logarithmic logic, recognizing thermal effects, and applying buffer principles, individuals transform abstract concepts into practical power—making accurate acid-base understanding not just a scientific skill, but a vital tool for troubleshooting, innovation, and stewardship in our increasingly quantified world.
Related Post

Unveiling The Mystery Of The Blue People Of West Virginia

What Is Katt Williams Net Worth? A Comprehensive Insight into the Comedian’s Financial Journey

Pifox Seamericau002639sse Newsroom Delivers Critical Updates Shaping Central U.S. Media and Financial Landscape

Where Is Mount Rushmore: America’s Monument Carved in Stone

