Periods Show Surprising Unity: Electron Energy Levels Collide in Electromagnetic Harmony
Periods Show Surprising Unity: Electron Energy Levels Collide in Electromagnetic Harmony
When atoms from different chemical families align on the periodic table, their shared electron energy levels reveal a fundamental elegance in atomic physics. Despite vast differences in atomic size, electronegativity, and chemical behavior, elements and even multiple period groups can exhibit electrons confined to nearly identical energy states—opening a window into deeper quantum symmetries. This phenomenon, where disparate elements converge electronically within the same or closely spaced electron shells, reshapes our understanding of atomic structure and its practical applications in chemistry and materials science.
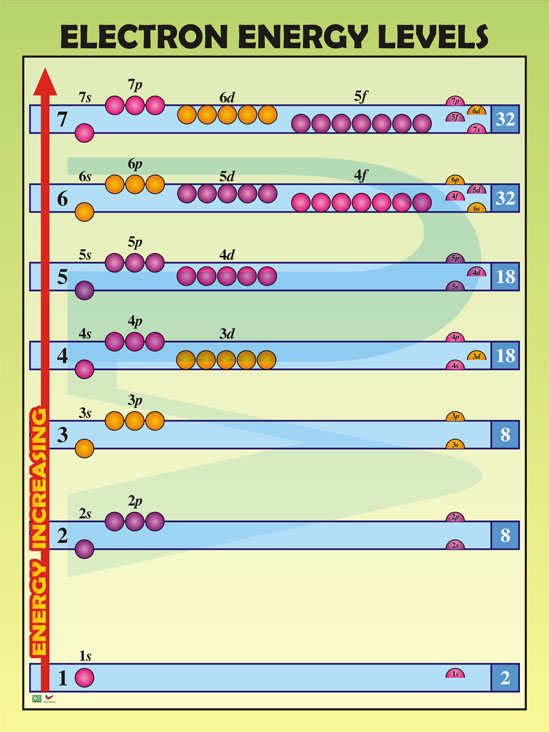
Understanding electron energy levels is foundational to atomic theory. Electrons occupy discrete quantized states around the nucleus, arranged in orbitals defined by energy, angular momentum, and magnetic quantum numbers. Each energy level corresponds to principal quantum numbers (n = 1, 2, 3, ...), with sublevels (s, p, d, f) further dividing those shells.
While period boundaries on the periodic table loosely correspond to principal quantum increases—group 1 (s-block) elements typically span periods 2 and 3, and group 17 (halogens) occupy period 4—their core valence electrons often reside in orbitals with nearly identical energies.
Take the alkaline earth metals (Group 2) and transition metals like chromium and copper (period 4). Despite divergent chemical properties, the 3d and 4s orbitals in elements such as zinc (Zn), cadmium (Cd), and chromium (Cr) exhibit overlapping energy profiles.
“The 4s orbital fills slightly before 3d,” explains Dr. Elena Marquez, a quantum atomic physicist at the Institute for Elemental Dynamics. “Yet within the same energy band, electrons occupy states with energy differences too small to measure with ordinary spectroscopy—but large enough to drive parallel chemical reactivity.” This subtle energy convergence facilitates electron sharing in bonding and enables catalytic activity across element groups.
The periodic table’s architectural logic hinges on shell organization, where each row reflects a new electron shell. Yet Sommerfeld’s quantum model reveals that subshells within a single shell—especially d and f orbitals—can host electrons with nearly identical energies, even across different periods. “Electron energy levels aren’t strictly confined to n values,” notes Dr.
Marquez. “The j quantum number and spin coupling create degenerate—or nearly degenerate—states that override principal quantum effects in heavy elements.” This insight explains why elements like iron (Fe, period 4) and manganese (Mn, period 5) display similar oxidation states and magnetic behavior despite being on separate periods. Their 3d subshell electrons occupy energy zones so close that transitions produce comparable spectral signatures and coordination chemistry.
Real-world implications emerge in advanced materials and catalysis. Nanoparticles doped with platinum-group metals exhibit precisely tuned d-orbital energies due to shared electron states across periods. These shared energy landscapes lower activation barriers in fuel cell reactions, enhancing efficiency.
In biomimetic enzyme design, synthetic metal centers replicate natural catalysts by exploiting cross-period energy similarities—showcasing how quantum electrostatic environments dictate reactivity.
Further illustrating this convergence, barium (Ba, Group 2) and titanium (Ti, Group 4) share valence electron configurations involving 4s² 3d¹⁰ in Ba vs. 4s² 3d⁵ in Ti, yet both stabilize +2 ions effortlessly.
This parallels modern quantum chemistry models suggesting that electron localization is not solely governed by period but by atomic electron correlation effects amplified at intermediate atomic numbers. Computational simulations now confirm: energy level spacing for valence orbitals across period 3 and beyond converges within a 1–3 eV band—plausible enough to influence redox potentials and ligand binding.
For researchers probing quantum confinement, molecular bonding, and sustainable energy, recognizing electrons with same energy levels across periods transforms empirical observation into actionable insight.
It underscores a core principle: while the periodic table neatly categorizes elements by atomic number, atomic electrons obey a subtler order—one where energy alignment, not mere period, unlocks chemical kinship. This dynamic reveals that elemental identity is not just structural but energetic, with profound consequences for next-generation materials and predictive modeling in chemistry.
The convergence of electron energy levels across distinct periods and groups exemplifies nature’s precision—a harmony written in quantum numbers.
By decoding this alignment, scientists extend their mastery over electron behavior, turning the periodic table from a static chart into a living map of atomic possibility.


Related Post

What Is Crystal Meth? The Deadly Reality Behind Methamphetamine’s Crystal Form

Is Lili Reinhart Really Related to Brittany Murphy? A Closer Look at the Unlikely Connection

Frankie Valli: Height, Voice, and the Enduring Legacy of theSocket

How Inertia of Rod Shapes Dynamics: The Unseen Force Governing Motion

