Molar Mass of Mercury: The Metal with a Dense, Diverse Identity
Molar Mass of Mercury: The Metal with a Dense, Diverse Identity
Mercury, the only metal that flows as a liquid at ambient temperature, holds a molar mass of 200.59 g/mol—fairly light for an element with such peculiar physical traits and a storied place in science, industry, and history. This precise molecular weight underpins Mercury’s behavior in chemical reactions, thermodynamic properties, and its management in modern applications. Understanding its molar mass is key to unlocking why this element behaves unlike any other in the periodic table.
With an atomic mass of approximately 200.59 atomic mass units (amu) per mercury atom—composed of 80 protons and typically 80 neutrons or one isotope being stable at ambient conditions—the molar mass reflects the sum of these atomic contributions. This value isn’t arbitrary; it governs density, vapor pressure, and phase transitions critical to mercury’s unique status. Mercury’s liquid state at room temperature—rare among metals—gives its atomic arrangement distinct spacing, directly influencing how mass and molecular weight interplay in physical systems.
The Role of Atomic Composition in Molar Mass
Mercury’s molar mass stems from its atomic structure: atomic number 80 places it as the 80th element, defined by 80 electrons and a core dominated by 80 neutrons in the most stable isotope, Hg-202 (though Hg-202 is minor; the shift to +80 neutrons is important).The interplay of protons and neutrons creates a heavy nucleus that significantly shapes the element’s mass. “The molar mass of mercury reflects not just addition, but the nuanced balance of nuclear stability and electron cloud distribution,” explains Dr. Elena Marquez, materials scientist at the Institute for Heavy Element Research.
- Mercury’s 80 protons define its identity, - Its variable neutron count (peaking at 122 for heavier isotopes) affects isotopic distribution, - Electron configuration influences chemical bonding and solid-liquid equilibrium. Despite slight isotopic diversity, the widely accepted molar mass of 200.59 g/mol serves as the benchmark for industrial, environmental, and laboratory calculations.
Physical Properties Influenced by Molar Mass
Mercury’s molar mass directly dictates key physical characteristics that define its real-world uses and risks.With a density nearly 13.5 times that of water—far exceeding copper and gold—its mass per unit volume stems from tightly packed atomic nuclei and moderate atomic radius. The 200.59 g/mol value correlates with a melting point of –38.83°C and boiling point of 356.7°C, properties that allow mercury to remain liquid in glass tubes while vaporizing at moderate heat. <
- Thermometers use its uniform expansion and density for accurate temperature readings. - Liquid light-emitting diodes (LEDs) and nuclear reactors exploit mercury’s photochemical and nuclear properties. Even small variations in molar mass across isotopes influence reaction kinetics—used in analytical chemistry for isotope ratio mass spectrometry, enabling dating and environmental tracing.
Isotopic Composition and Its Impact on Molar Mass
Though mercury has only one stable isotope under standard conditions—Hg-202—the trace presence of others, such as Hg-199, Hg-200, and Hg-204, introduces minor isotopic variations. The most abundant isotope, Hg-200, accounts for roughly 54% of natural mercury. Despite these isotopic shifts, the molar mass remains stable at 200.59 g/mol, calibrated with high precision by spectroscopic measurements and mass spectrometry.<
This consistency underscores the reliability of using a fixed molar mass in engineering and environmental assessments.
Environmental and Health Considerations
Mercury’s molar mass plays a silent but critical role in environmental and toxicological assessments. Its density and liquid form enhance bioaccumulation in aquatic ecosystems—especially as methylmercury, a neurotoxin.While the specific mass per atom doesn’t alter toxicity, understanding mercury’s molecular weight aids in modeling its dispersion in air, water, and food chains. Mercury’s volatility—governed by intermolecular forces influenced by atomic mass—dictates emission risks. At 356.7°C boiling point, heated mercury evaporates, releasing vapor that travels globally.
“Molar mass helps predict phase changes and atmospheric transport,” says Dr. Marquez. “It’s not just a number—it’s a bridge between chemistry and public health.”
Industrial Management and Regulatory Frameworks
Globally, mercury use is tightly regulated due to its toxicity and persistent environmental impact.The 200.59 g/mol value is embedded in measurement standards, emission limits, and waste treatment protocols. Organizations such as the World Health Organization and the U.S. Environmental Protection Agency rely on precise molar data to enforce safe exposure levels and manage contaminated sites.
Regulatory limits—such as mercury emissions in coal-fired power plants—hinge on accurate mass concentrations derived from this molar mass. Technical probes and calibration standards use mercury’s known weight for precision, ensuring compliance across industries. “Mercury’s mass is a silent sentinel in policy,” observes Dr.
Tran. “It underpins global efforts to reduce exposure while balancing utility.”
Across science, engineering, and environmental science, the molar mass of mercury stands as a fundamental constant—precise, consistent, and profoundly impactful. It shapes how we measure, manage, and mitigate one of nature’s most peculiar elements, ensuring safety, innovation, and understanding remain grounded in reality.



![[ANSWERED] optional Mercury chloride is a fungicide If the molar mass ...](https://media.kunduz.com/media/sug-question-candidate/20211012211744959001-3659380.jpg?h=512)
Related Post
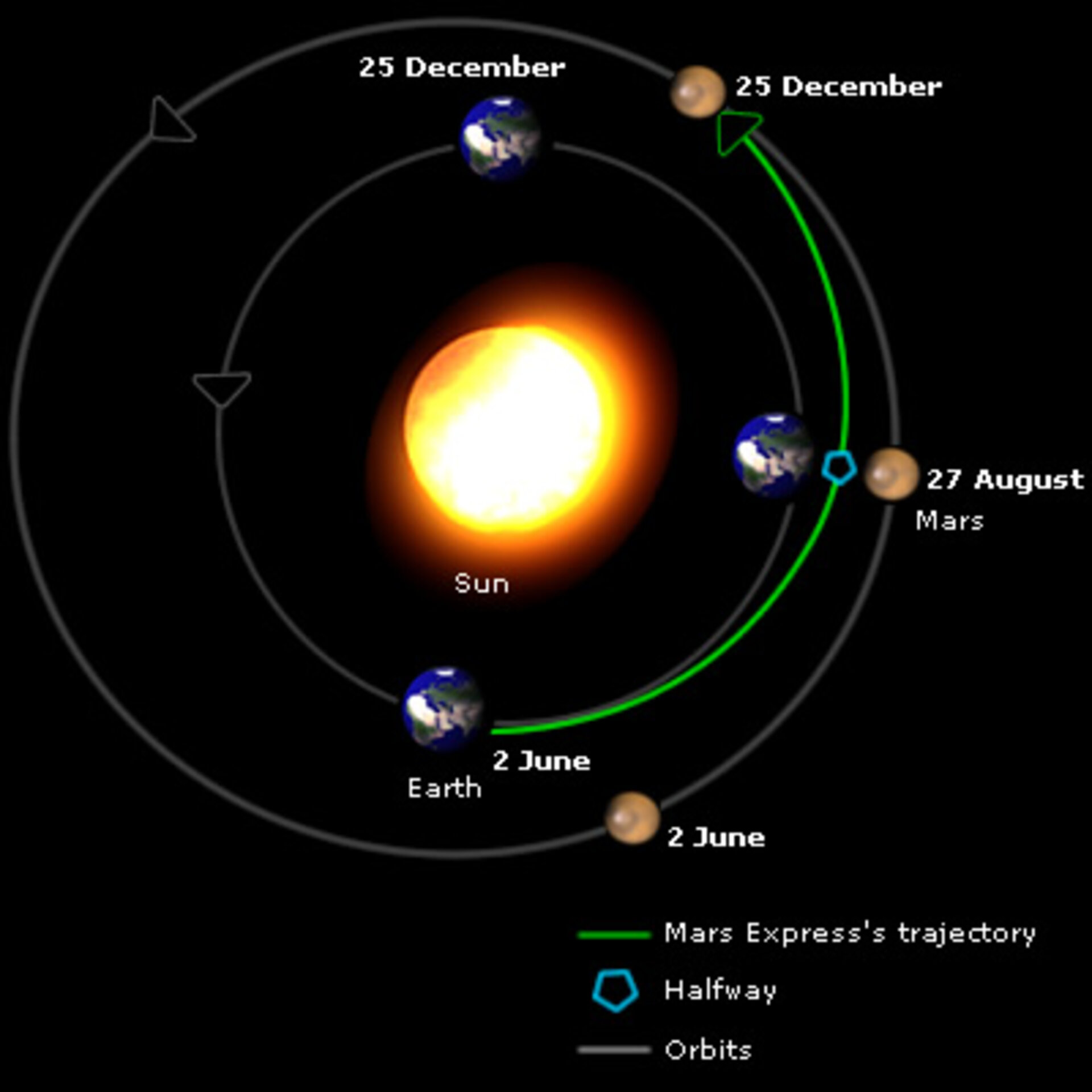
How Far Apart Are Earth and Mars? Unraveling the Dynamic Distance Between Our Two Planetary Neighbors

MMBC Tour & Travel Login: Your Gateway to Streamlined Travel Management

Kelsey Plum’s Love Life Under the Spotlight: Inside Her Relationship with Her Boyfriend

University of Phoenix 2024 Disbursement Dates: Key Dates and Financial Roadmap for Students

