Mastering the Stoichiometric Equation: The Stoich Chart That Defines Chemical Precision
Mastering the Stoichiometric Equation: The Stoich Chart That Defines Chemical Precision
At the heart of chemical science lies stoichiometry—the quantitative relationship between reactants and products in a chemical reaction. A well-constructed Stoich Chart transforms complex molecular interactions into a visual, logical framework, enabling chemists and students alike to decode reaction ratios with clarity. This powerful tool, often treated as a mere chart, is in fact a foundational instrument that bridges symbolic equations to real-world applications, offering both accuracy and insight.
From balancing equations to predicting yields, the Stoich Chart streamlines solutions that once required tedious manual calculations. Its structured format reveals molar relationships, leverages conservation of mass, and serves as a gateway to deeper understanding of chemical transformations.
Decoding the Stoich Chart: Structure and Function
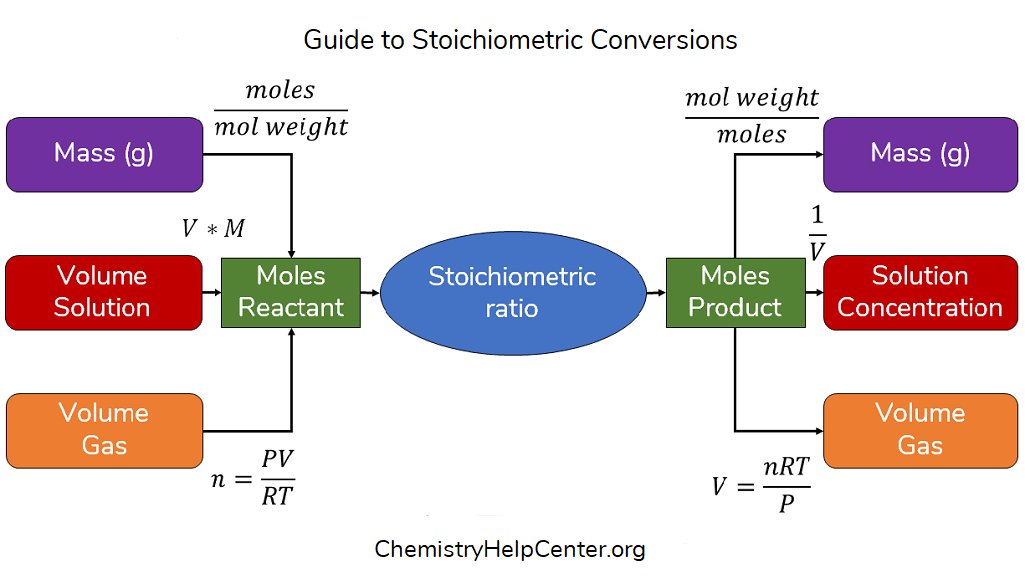
The Stoich Chart is more than a table—it is a systematic breakdown of atomic quantities in chemical equations. At its core, it displays reactants on the left and products on the right, with coefficients showing exact molar ratios.
Each element’s symbol, number of atoms, and subscript counts reflect conservation laws, ensuring no atom is hidden or created. Unlike generic equation annotations, the Stoich Chart compiles this data in a consistent, scalable matrix.
Key components:
- Reactants: Listed left with formulas and coefficients indicating starting material quantities.
- Products: Placed right with formulas and coefficients denoting final outcomes.
- Molar Ratios: The ratios of coefficients define conversion factors between substances.
- Conservation of Mass: Total atoms on each side match, validating reaction fidelity.
- Subscripts and Superscripts: Reflect molecular identity and charge state where applicable.
This precision ensures every reaction—from combustion to synthesis—can be analyzed with exactness, removing ambiguity from what could otherwise be a puzzle of molecular proportions.
The Stoich Chart in Action: From Theory to Transformation
To grasp the practical power of the Stoich Chart, consider the combustion of methane: CH4 + 2O2 → CO2 + 2H2O. A Stoich Chart for this reaction specifies: – Reactants: 1 CH4 (4 hydrogen atoms, 1 carbon) and 2 O2 (2 oxygen atoms each), totaling 6 hydrogen and 2 carbon atoms.
– Products: 1 CO2 (1 carbon, 2 oxygen) and 2 H2O (4 hydrogen, 2 oxygen), summing to 5 hydrogen and 4 oxygen. Yet the chart traces both mass balance and stoichiometric ratios clearly: 1 C : 1 C, 4 H : 4 H, and 2 O : 2 O—confirming conservation. ">> *“The Stoich Chart doesn’t just balance equations—it reveals the story of atoms moving through a reaction,”* says Dr.
Elena Marquez, a chemical education specialist. This narrative strength turns data into understanding.
This visual mapping extends beyond simple combustion. In a synthesis reaction like ammonia production: N2 + 3H2 → 2NH3, the chart shows nitrogen contributes 2 atoms in products while hydrogen triples—highlighting the 3:1 ratio essential for scaling industrial processes efficiently.
Even complex multi-step reactions rely on Stoich principles to track atom flow across intermediate stages.
Applications Across Disciplines: From Lab to Industrial Scale
The Stoich Chart’s utility spans academic, research, and industrial spheres. In high school labs, students use it to predict reaction outcomes before mixing chemicals, minimizing waste and enhancing safety. In pharmaceutical development, stoichiometric accuracy ensures precise reagent ratios critical for yield optimization and cost control.
">> *“Without a clear Stoich Chart, a missed coefficient isn’t just a typo—it’s a potential explosion in industrial reactors or wasted drug batches,”* notes chemical engineer Rajiv Patel. Environmental chemistry benefits, too: stoichiometry underpins calculations for pollutant neutralization, where exact reagent amounts prevent secondary contamination.
Even nuclear chemistry employs stoichiometric logic—though not involving electrons, the principle of mass-energy balance follows the same conservation ethos.
From balancing lab equations to modeling cosmic fusion, the chart remains an indispensable analytical anchor.
The Stoich Chart as a Pillar of Chemical Education
In classrooms worldwide, the Stoich Chart is a cornerstone of chemistry pedagogy. It turns abstract relationships into tangible ratios, making stoichiometry accessible to students navigating bonds, moles, and molarity. Each coefficient is a clue, each row a section of a larger puzzle.
Teachers use it to reveal common errors—imbalanced equations, overlooked atoms—processing misunderstandings into teachable moments. ">> “Students often see coefficients as numbers, but the Stoich Chart teaches them meaning,”* explains high school teacher Maria Chen. “They stop calculating gas volumes in formulas and start seeing chemistry as a coherent system.” This shift from computation to comprehension fosters deeper engagement, preparing future scientists not just to compute, but to reason through molecular interactions.
Digital tools further enhance the chart’s reach.
Interactive Stoich Chart builders allow real-time coefficient adjustments, instantly validating conservation laws and visualizing yield impacts. These platforms, used in virtual labs and remote learning, democratize access to chemical precision, proving the Stoich Chart evolves with technology—remaining timeless in purpose yet modern in delivery.
From balancing equations to optimizing industrial processes, the Stoich Chart is not merely a figure—it is a framework for chemical reasoning. It transforms chemical symbols into a story of atomic transformation, ensuring balance, accuracy, and insight at every scale.
In mastering the Stoich Chart, chemists grasp more than ratios: they understand the language of materials and reactions that shape our world.

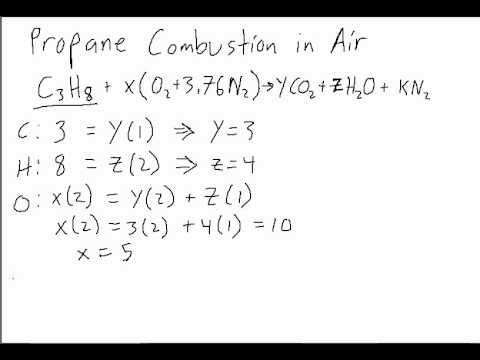

Related Post

Unblock the Storm: Snow Rider 3D Unblocked Lights a New Era in Immersive Riding Games
What Is Nonsa? Decoding the Emerging Force in Healthcare SaaS

The Sisters of Hollywood: Julia Roberts’ Quietly Remarkable Sibling Legacy Beyond the Screen

Exploring The Life And Age Of Alexandra Grant: From Early Promise to Poised Public Voice

