How Deep Brain Stimulation Rewires the Brain: The Neuroscientific Breakthrough Reshaping Therapy
How Deep Brain Stimulation Rewires the Brain: The Neuroscientific Breakthrough Reshaping Therapy
Deep brain stimulation (DBS) has long been a frontier in neuroscience, offering transformative potential for patients with neurological disorders such as Parkinson’s disease, epilepsy, and treatment-resistant depression. Far more than a simple electrical intervention, DBS acts as a neuromodulatory force that reshapes aberrant neural circuits through precise, targeted impulses. Recent neuroscientific advances reveal the intricate biological mechanisms behind its efficacy—revealing not just how it works, but why it can reverse pathological brain activity with remarkable specificity.
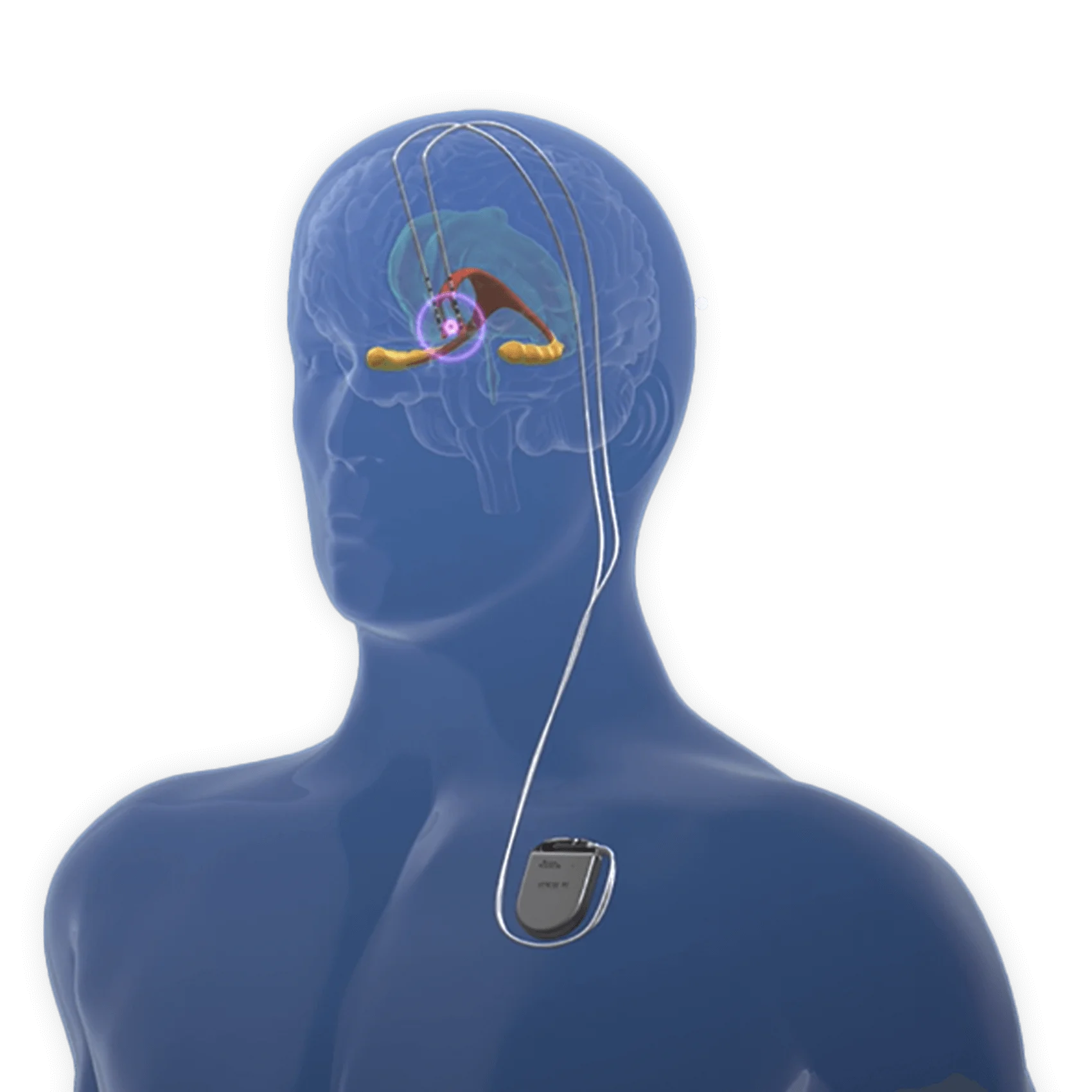
By interfacing directly with dysfunctional networks, DBS disrupts pathological synchrony and restores balanced neural communication, fundamentally altering the brain’s functional architecture. At the cellular level, DBS exerts its influence primarily through controlled depolarization of key neuronal populations. Unlike destructive treatments, DBS delivers high-frequency electrical pulses—typically 130–185 Hz—that induce a “pacing-like” effect in targeted nuclei such as the subthalamic nucleus (STN), ventral intermediate nucleus of the thalamus (Vim), or the nucleus accumbens (NAc).
These electrical signals do not incinerate tissue but instead modulate action potential firing patterns, suppressing hyper-synchronized oscillations characteristic of disorders like Parkinson’s. “The key is not just stimulation, but dynamic tuning—reshaping misfired circuits,” explains Dr. Elena Petrova, a neurophysiologist at the University of Oxford.
Each pulse acts as a temporal filter, filtering out pathological neural “noise” while permitting healthy signal flow across connected regions. This modulation has measurable effects on neural connectivity. Functional MRI and pre-stimulation mapping reveal that DBS transiently alters connectivity patterns within the motor and limbic networks, effectively resetting dysfunctional loops.
In Parkinson’s patients, for example, DBS of the STN dampens excessive beta-band oscillations (13–30 Hz) in the basal ganglia, which are strongly associated with motor rigidity and bradykinesia. "When DBS suppresses pathological synchronization, downstream areas like the motor cortex receive normalized input—triggering a cascade of functional recovery," notes Dr. Marco Lin, neurosurgeon and DBS investigator at Johns Hopkins.
Beyond oscillatory control, DBS influences neurochemical signaling and plasticity. Stimulation alters the release of neurotransmitters such as dopamine, glutamate, and GABA, realigning excitatory-inhibitory balance critical for circuit stability. This neuromodulatory effect supports long-term synaptic plasticity, reinforcing adaptive neural patterns.
“DBS isn’t just a band-aid,” says Dr. Lin. “It’s a catalyst for reorganization—encouraging the brain to rewire itself over time.” Studies using animal models and clinical imaging confirm increased levels of growth factors such as BDNF (brain-derived neurotrophic factor) following DBS, promoting neuron survival and axonal sprouting in targeted regions.
The spatial precision of DBS is a critical factor in its success. Traditional lesion therapies affected broad brain regions with collateral damage, but modern DBS employs directional leads and closed-loop systems that deliver stimulation only when pathology is detected. “Adaptive DBS systems sense abnormal activity in real time and respond with tailored pulses—reducing off-period symptoms like freezing in Parkinson’s,” explains neuroengineer Dr.
Sarah Chen. This closed-loop capability enhances therapeutic efficacy while minimizing side effects, a breakthrough supported by recent trials demonstrating improved motor control with reduced off-time. Emerging research extends DBS beyond motor disorders into mental health.
Targeting circuits in the NAc and anterior cingulate cortex, DBS shows promise in treatment-resistant depression and OCD by normalizing hyperactive limbic activity. Neuroimaging during DBS in depression patients reveals disinhibition of prefrontal regions involved in mood regulation, correlating with clinical improvement. “We’re moving into a new era where brain circuits are calibrated, not just suppressed—offering hope for conditions once deemed intractable,” states Dr.
Petrova. Technological innovation continues to refine DBS delivery. Microelectrode arrays now enable recording of single-unit activity during surgery, allowing surgeons to validate optimal stimulation sites in real time.
Wireless, implantable pulse generators reduce infection risk and improve patient comfort. Meanwhile, machine learning algorithms parse neural data to predict optimal stimulation parameters, personalizing therapy with unprecedented granularity. “This convergence of neuroscience, engineering, and data science is propelling DBS from a stabilization tool to a true network reprogramming device,” emphasizes Dr.
Lin. Yet challenges persist. Individual variation in brain anatomy and pathology means responses remain highly personalized.
Long-term effects on neural plasticity require ongoing study, especially with newer adaptive systems. Ethical considerations around autonomy and neural control also demand careful scrutiny. Nevertheless, the neuroscience is clear: DBS works by recalibrating dysfunctional networks—not overriding them.
It leverages the brain’s intrinsic capacity to adapt, offering a biologically grounded pathway to functional recovery. The broader implication is profound. By harnessing targeted electrical modulation, DBS exemplifies a new paradigm in medicine—one where precise neuromodulation restores brain function at a circuit level.
As research advances, the potential applications of DBS may expand into neurodegenerative diseases, chronic pain, and cognitive decline, marking a pivotal shift in how we treat neurological and psychiatric conditions. The deeper one ventures into the mechanics of DBS, the more apparent it becomes: it is not merely stimulating the brain, but communicating with it—re-establishing coherence in the symphony of neurons. Through this lens, deep brain stimulation emerges not as a robotic intervention, but as a sophisticated dialogue between technology and biology, one that holds the promise of redefining healing in the 21st century.
The Cellular Dance: How DBS Alters Neuronal Activity
At the microscopic level, DBS triggers a complex interplay of electrical forces that reshape how neurons communicate. Each stimulation pulse delivers brief bursts of current that depolarize neuronal membranes, pushing them past threshold and initiating action potentials. But the effect goes beyond individual cells—neural circuits function as interconnected networks, and DBS disrupts pathological synchrony without causing widespread excitation.Controlled, high-frequency stimulation prevents excessive gamma-band activity, which underlies many movement disorders. This precise timing suppresses silence-like bursts in the basal ganglia and restores rhythmic coordination across pathways. As Dr.
Chen observes, “DBS functions like a conductor adjusting tempo—allowing harmony rather than cacophony.” Key Mechanisms in Action: - Degradation of pathological beta oscillations in Parkinson’s patients through frequency-specific inhibition. - Enhanced phasic firing in motor circuits, improving signal initiation. - Modulation of neurotransmitter release timing to recalibrate excitatory-inhibitory balance.
- Promotion of local synaptic plasticity via BDNF upregulation and dendritic remodeling.
Targeting Circuits with Precision
DBS efficacy hinges on targeting specific nuclei connected to global brain networks. The subthalamic nucleus (STN), for instance, regulates the basal ganglia's output to the motor thalamus; stimulating it dampens abnormal bursting.Similarly, the ventral intermediate nucleus (Vim) modulates tremor by disrupting rhythmic firing in cerebellar circuits. Advances in tractography and intraoperative mapping now allow surgeons to visualize fiber pathways, guiding electrode placement with sub-millimeter accuracy. “We’re no longer just pointing in the right direction—we’re hitting the exact node that reroutes pathology,” says Dr.
Petrova. Clinical Evidence of Neuroplastic Change: Neuroimaging studies confirm DBS induces measurable plasticity. Functional MRI shows reduced hyperactivity in motor cortices during task execution post-stimulation, while diffusion tensor imaging reveals strengthened functional connectivity between prefrontal and limbic regions in depression cases.
Longitudinal data suggest these circuit-level changes persist beyond acute stimulation, supporting improved clinical outcomes over months or years. This durability underscores the distinction between temporary suppression and lasting brain network reformatting.
Adaptive Systems: The Future of Personalized Neuromodulation
The next generation of DBS devices moves beyond fixed settings to closed-loop systems that sense and respond to real-time neural activity.These adaptive platforms detect abnormal patterns—such as the beta bursts in Parkinson’s—and deliver stimulation only when needed, minimizing energy use and side effects. “This approach respects the brain’s natural rhythms, intervening only in pathological deviations,” explains Dr. Lin.
Clinical trials report reduced off-periods by 30–50%, with fewer side effects than conventional DBS. As machine learning interprets complex neural signatures, therapy becomes increasingly anticipatory and individualized. Emerging Applications Beyond Movement Disorders: While DBS is established in neurosurgery, research now explores its potential in psychiatric and cognitive disorders.
Targeting the nucleus accumbens shows promise in treatment-resistant depression, dampening hyperactive reward circuitry. Stimulation of the anterior cingulate cortex may alleviate obsessive-compulsive symptoms by normalizing hyperconnectivity between frontal and subcortical regions. Early imaging and behavioral studies suggest DBS does not erase identity but realigns dysfunctional networks, restoring emotional and cognitive equilibrium.
Technological and Ethical Frontiers: Miniaturization of hardware and wireless data transmission enable fully implantable, self-regulating systems. However, ethical questions arise around autonomy, identity, and long-term neural adaptation. Precise targeting reduces off-target effects, but personalized responses demand rigorous validation.
As the field evolves, collaboration between neuroscientists, engineers, and clinicians remains essential to ensure safety, accessibility, and equitable use. The neuroscientific basis of DBS confirms it as more than a treatment—it is a dynamic tool for brain reorganization, capable of reshaping neural circuitry with astonishing specificity. By merging electrical engineering with deep biological insight, DBS redefines the boundary between human neural potential and technological intervention.
As research accelerates, the prospect of restoring function across a spectrum of disorders grows ever more tangible, heralding a new era in which the brain’s own plasticity becomes the ultimate healing engine.
Related Post

Neuroscientifically Does “Embodied Cognition” Actually Exist?

iPhone 15: Simple Guide to Changing Your SIM Card – Upgrade with Confidence

November in Virginia Beach: Mild Rain, Coastal Breezes, and Unpredictable Coastal Meteo

