Gold Number Of Protons: The Invisible Code Behind Atomic Identity
Gold Number Of Protons: The Invisible Code Behind Atomic Identity
There are three fundamental numbers etched into every atom: electrons, neutrons, and protons — but only the number of protons defines the element itself. Numbered precisely between 1 and over 118, the Gold Number of Protons — a lesser-known metric — reveals the precise identity of an element with mathematical rigor, offering insight into its atomic behavior, chemical properties, and place in the periodic table. Far beyond a mere count, this number acts as a foundational key to understanding atomic structure, stability, and the very fabric of matter.
The Gold Number of Protons equals a simple count: the total number of positively charged protons residing in an atom’s nucleus. For hydrogen, it is 1 — a deceptively simple foundation for all chemistry. As one scientist noted, “Protons are the architects of atomic identity; without them, the periodic table would lose its coherence.” This singular count determines atomic number, chemistry, and even the behavior of elements in reactions, fusion, and stellar nucleosynthesis.
The number 1 defines hydrogen, 2 defines helium, 6 defines carbon, and anomalies like golden primes in heavier elements reveal complexity beneath simplicity.
Each element’s Gold Number directly influences electron configurations and chemical versatility. For example, carbon’s atomic number of 6 means six protons, yielding an electron arrangement critical for forming stable covalent bonds — the backbone of organic chemistry.
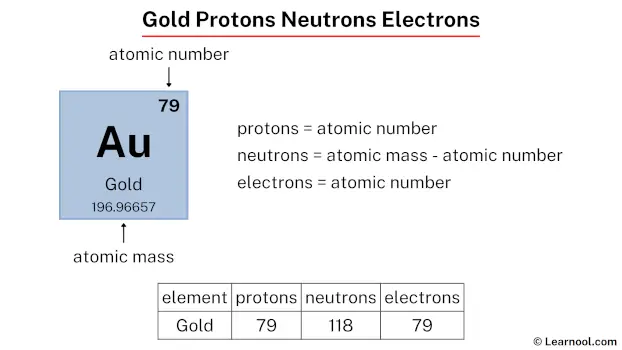
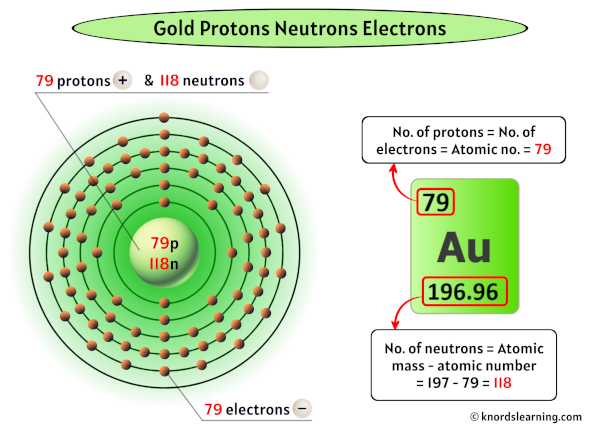
Without this precise proton count, carbon would not support life’s intricate molecular structures. Similarly, gold itself, with 79 protons, exhibits unique electron shielding and relativistic effects that alter its density, luster, and resistance to corrosion — properties directly traceable to its proton number. Understanding the Gold Number: More Than Just Atomic Counting Numbering every element by protons transforms abstract atomic theory into an actionable scientific tool.
While atomic mass reflects weighted proton-neutron sums, the Gold Number stands alone as a unique identifier. Consider oxygen: atomic number 8, implying eight protons. Each proton contributes to a nucleus that, under electric repulsion, influences how eight electrons arrange in orbit — determining oxygen’s role as a vital oxidizer and essential breathable gas.
Mathematically, the Gold Number is not probabilistic but deterministic. It forms the basis of atomic tables, nuclear physics models, and spectroscopic analysis. For instance, isotopes of an element share the same Gold Number, yet differ in neutron count — preserving elemental identity while varying stability.
Silver (47 protons) and gold (79) remain distinct despite differences in neutron profiles. This constancy enables scientists to track elements reliably across reactors, stars, and laboratories. The Periodic Table, Redefined: Protons as Constructive Units Modern periodic classification hinges on atomic number — fundamentally the Gold Number.
Dmitri Mendeleev’s original table evolved as new elements were discovered, all confirmed by verification of proton counts. The Gold Number thus serves both historical continuity and predictive power: predicting chemical behavior, bonding patterns, and even material properties. Take fluorine (9 protons), the most electronegative element — its proton count directly enables a vast array of ionic compounds and industrial applications.
Gold Number also reveals intriguing patterns across the periodic table. Elements in Group 1 (alkali metals) feature 1 proton — a value linked to single valence electron and high reactivity. Transition metals like iron (26) or gold (79) exhibit higher proton counts, enabling complex electron shells and catalytic roles prized in industry.
Even superheavy elements, such as oganesson (118 protons), derive identity from this core count, though their instability underlines the limits of nuclear stability governed by proton-proton electrostatic forces. Civil and Technological Implications of Proton Count Beyond pure science, the Gold Number impacts technology and innovation. Nuclear engineering uses precise proton counts to model fission chain reactions, where changes in proton number induce shifts in atomic stability and energy release.
In materials science, proton-rich elements like phosphorus (15) enable bioluminescence and organic electronics. Meanwhile, in fusion research, targeting nuclei with specific proton numbers raises the potential to replicate stars’ energy processes on Earth.
Historically, measuring proton counts transformed chemistry.
In the 1800s, John Newlands and later Henry Moseley leveraged atomic number — still rooted in proton count — to order elements accurately, laying groundwork for quantum theory. Today, advanced spectrometers continuously refine proton numbers in exotic isotopes, pushing boundaries in nuclear physics and astrophysics.
Notably, the Gold Number also clarifies anomalies.
Radon (86 protons), for instance, balances high proton repulsion with relativistic electron effects, explaining its volatility. Similarly, gold’s 79 protons balance nuclear stability with unique catalytic qualities — differences measurable only through precise proton count.
In essence, the Gold Number of Protons is the silent signature of elemental identity.
It defines atomicity, drives chemistry, and shapes technology — from ancient metallurgy to quantum computing. As science explores new frontiers from synthetic elements to neutron stars, this number remains an unwavering anchor, grounding the complexity of matter in simple yet profound precision. Every proton counts — not just in atoms, but in the legacy of discovery itself.


Related Post

Discover The Secrets Of Torque-to-Yield Bolts: How Precisionly Tightening UnlOCKs Industrial Efficiency

Cole Maness, Erika Christensen, and Polly At: Shaping Modern Discourse on Identity, Education, and Narrative Complexity

Itù: The Hidden Engine Transforming African Markets Through Localized Innovation

Exploring Rossif Sutherland Movies And Tv Shows

