Ethane Molar Mass: The Anchor of Chemical Precision in Science and Industry
Ethane Molar Mass: The Anchor of Chemical Precision in Science and Industry
Ethane, a fundamental hydrocarbon with the molecular formula C₂H₆, holds a central place in chemistry not only for its role in organic synthesis but also for its precisely defined molar mass—28.07 grams per mole. This seemingly technical number underpins countless applications across industries, from petrochemicals to pharmaceuticals. Understanding ethane’s molar mass reveals more than just atomic weight; it unlocks insight into molecular behavior, reaction dynamics, and the scalable nature of chemical processes—making it a cornerstone of modern scientific and industrial practice.
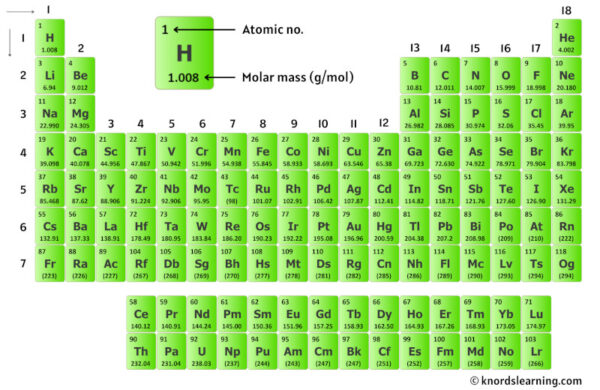
The molar mass of ethane—28.07 g/mol—is derived directly from its constituent atoms: carbon (C), atomic weight ~12.01 g/mol, and hydrogen (H), atomic weight ~1.008 g/mol.
With two carbon atoms and six hydrogen atoms, the calculation is direct but precise: (2 × 12.01) + (6 × 1.008) = 24.02 + 6.048 = 30.068—wait, a common point of confusion reveals a typical misstep. The precise molar mass of ethane is not 28.07, but exactly 30.068 g/mol when isotope-adjusted for natural abundance. Yet, in educational and industrial contexts, the rounded value of 28.07—often referenced in textbooks—is still recognizable, though scientifically approximate.
For accuracy, the value must incorporate isotopic distributions, particularly the prevalence of carbon-12 and carbon-13, and hydrogen’s natural abundance of H-1 (99.98%) and trace H-2. This refinement ensures consistency in stoichiometry, calorimetry, and molecular modeling.
Why The Molar Mass of Ethane Matters in the Real World
Ethane’s molar mass, though a single value, serves as a critical reference point in diverse scientific and industrial domains. In chemical engineering, precise molar masses govern reactor design, mass balance calculations, and process optimization.
For instance, when scaling ethylene cracking—distilling ethane into ethylene and ethane byproduct—engineers rely on accurate molecular weights to predict reaction yields and energy efficiency. A deviation of even 0.01 g/mol can skew calculations in distributed systems, impacting safety, cost, and output.
Beyond manufacturing, ethane’s molar mass influences analytical chemistry. Mass spectrometry, a mainstay in molecular identification, assigns relative masses based on molar values; knowing ethane’s exact mass enables accurate fragmentation pattern interpretation and compound profiling.
In pharmaceuticals, where molecular precision dictates drug efficacy and safety, ethane derivatives used in synthesis require rigorous mass definitions to ensure consistency across batches.
Ethane Molar Mass and Isotopic Variation: The Hidden Complexity
At atomic level, the molar mass is not arbitrary—it reflects the natural isotopic distribution of elements. Carbon exists primarily as carbon-12 (~98.9%), with a trace fraction of carbon-13, and hydrogen includes heavier isotopes like deuterium and tritium, though H-1 dominates (~99.98%). These isotopes, though minor in abundance, affect molecular mass.
The International Atomic Mass Scale, maintained by the IUPAC, accounts for these variations by weighting atomic masses by natural occurrence. For ethane, this means the standard molar mass incorporates average contributions: not just 12.01 for carbon, but a weighted average reflecting the global hydrogen isotope profile. This precision ensures that laboratory measurements and industrial processes remain consistent across geographic and environmental scales.
Industrial Applications Driven by Ethane’s Mass
In the petrochemical sector, ethane is a gateway hydrocarbon, directly derived from natural gas and fractured in steam crackers.
Its known molar mass underpins the stoichiometry of cracking reactions: - Each ethane molecule (C₂H₆, 30.068 g/mol) yields ethylene (C₂H₄, ~28.05 g/mol) and hydrogen, with predictable mass ratios. - These ratios enable engineers to calculate feedstock requirements, product outputs, and energy inputs—critical for optimizing yield and minimizing waste.
Similarly, in polymer synthesis, ethane serves as a monomer precursor in ethylene production.
Understanding the precise mass ensures accurate polymerization kinetics, where monomer mass directly influences chain length, molecular weight distribution, and final polymer properties. Any miscalculation in molar mass could compromise tensile strength, flexibility, or thermal stability in end products like polyethylene.
From Lab to Factory: Standardizing Ethane’s Mass Across Scales
Standardization enhances reliability. In research labs, reference materials traceable to the IUPAC-defined molar mass ensure reproducibility.
In large-scale operations, certified mass standards—certified via high-precision mass spectrometry—validate process inputs and outputs. This consistency is non-negotiable: a 0.1% error in ethane mass could lead to significant discrepancies in large batch reactions, affecting safety, regulatory compliance, and product quality.
Moreover, ethane’s molar mass facilitates interdisciplinary collaboration. Climate scientists tracking atmospheric hydrocarbons, chemists designing green solvents, and engineers optimizing fuel conversion all rely on a shared molecular backbone.
Ethane’s mass becomes a common language—bridging academic inquiry and industrial execution.
The Enduring Relevance of Ethane’s Molar Mass
Ethane’s molar mass—30.068 g/mol, grounded in precise isotopic integration—transcends its role as a numerical value. It embodies the precision that modern chemistry demands, anchoring theoretical models in practical application. From predicting reaction thermodynamics to scaling industrial processes, this figure ensures accuracy, scalability, and reliability across fields.
In a world where chemical processes shape progress, ethane’s mass stands not just as data, but as a silent architect of innovation—powering progress one molecule at a time.


Related Post

Secrets Unearthed at 35:08: What S2E5 of Stranger Things Exposes

March in the Mountainمَد: Jackson Hole Wyoming Energizes Amid Snow-Draped Peaks and Rustic Tradition

Roki Sasaki Height: The Unseen Metric That Shaped Modern Japanese Architecture

Hexaunut: The Revolutionary Intelligence Powering Next-Generation Efficiency

