Decoding the Building Blocks: The Ptable Periodic Table Reveals the Science Behind Elemental Mastery
Decoding the Building Blocks: The Ptable Periodic Table Reveals the Science Behind Elemental Mastery
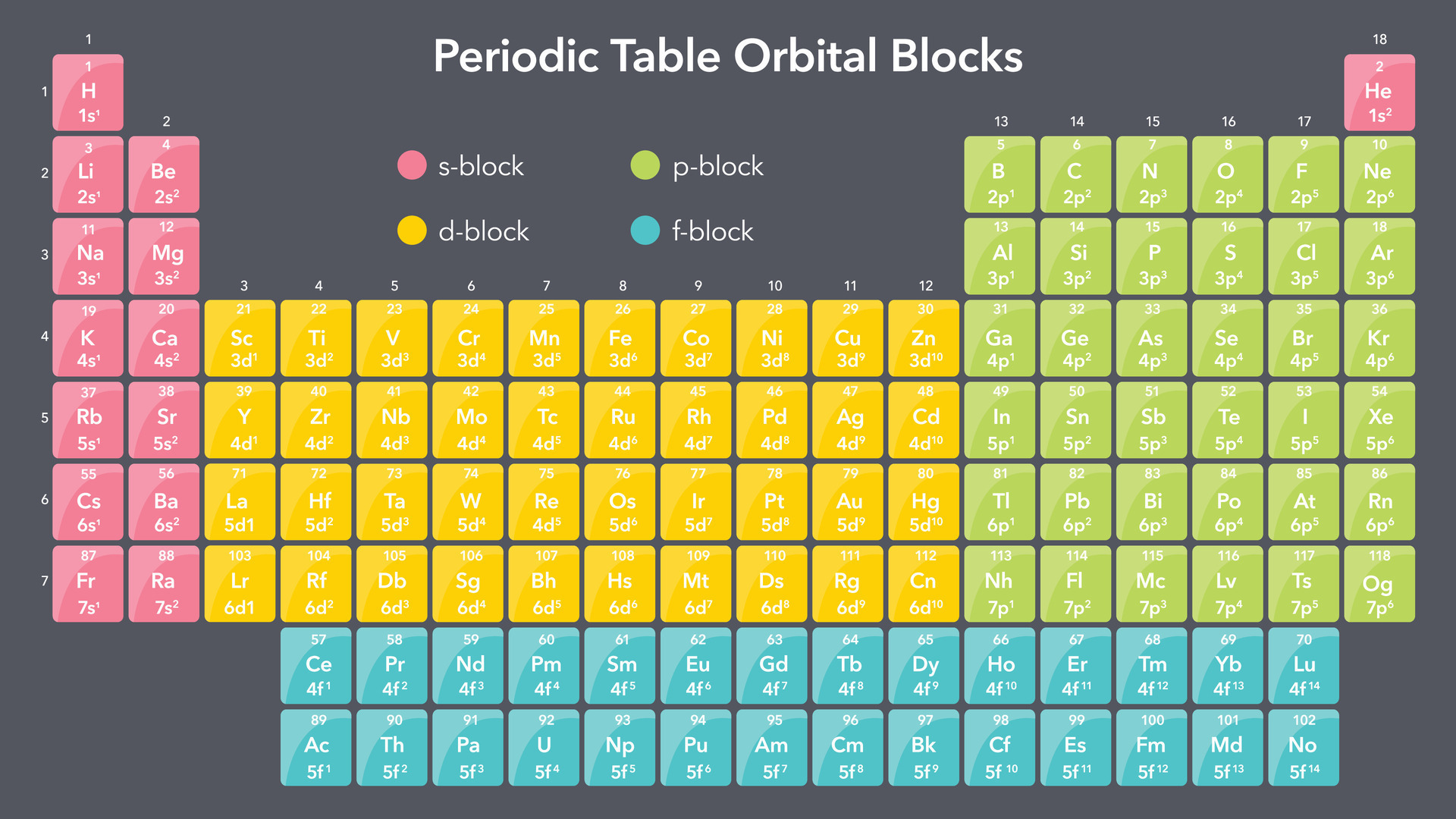
At the heart of chemistry lies the Ptable Periodic Table—a meticulously organized map of the elements that govern all matter on Earth. Spanning across seven periods and 18 groups, this iconic tool synthesizes atomic structure, electron configurations, and physical properties into a single, visually intuitive framework. Engineers, educators, and scientists rely on it daily not just as a reference, but as a foundational guide to understanding reactivity, material science, and the very nature of chemical bonding.
From hydrogen to uranium, every element told through its place and pattern offers insights that drive innovation across industries—pharmaceuticals, energy, nanotechnology, and beyond.
The Evolution of Element Classification: From Mendeleev to Ptable
The modern Ptable is the culmination of over 200 years of scientific observation and classification. Julius von Mendeleev’s 1869 periodic system, based on atomic mass and chemical behavior, laid the groundwork, but only with the 20th-century discovery of atomic number—championed by Henry Moseley—did the periodic table gain true predictive power. Today’s Ptable reflects advances in atomic theory, quantum mechanics, and computational modeling.
Each cell in the chart encodes critical data: atomic radius, electronegativity, ionization energy, and melting point. “The periodic table is not just a chart,” notes Dr. Catherine Bell, a condensed matter physicist.
“It’s a predictive engine powered by quantum principles.” The transition from Mendeleev’s crude rows to today’s electron-orbital-based layout exemplifies how deeper understanding transforms mere data into a live tool for discovery.
Core Groupings and Their Functional Significance
The periodic table’s structure reveals function: elements in the same group share valence electron configurations, dictating similar chemical behavior. Alkali metals (Group 1) ignite spontaneously and conduct electricity with relish, while halogens (Group 17) act as potent oxidizers. Transition metals (Groups 3–12) shine as catalysts and structural stalwarts, their variable oxidation states enabling complex redox reactions.
Rare Earth elements (lanthanides and actinides) remain crucial in modern electronics and green energy tech. \pi a clearer look at key group trends:
- Alkali Metals (Group 1): Exhibit 1 electron ionization, react violently with water. „Their reactivity decreases down the group due to increasing atomic radius,” explains chemistry professor Raj Patel.
„Cesium burns more explosively than lithium,” highlighting escalating reactivity trends.
- Chalcogens (Group 16): Oxygen stands out as a near-universal oxidizer, driving combustion and respiration alike. Sulfur and selenium serve dual roles in biological systems and industrial processes.
- Transition Metals: Iron, copper, and nickel form alloys and catalyze industrial synthesis—from ammonia production to catalytic converters—exemplifying their versatile chemical behavior.
Periodicity—the repetition of trends—lies at the core of the table’s utility. The 18 periods reflect increasing electron shells, while groups reorganize by valence shell electron count, revealing recurring patterns in ionization energy, electron affinity, and atomic size.
This periodicity allows scientists to predict how new, yet-to-be-discovered elements might behave, guiding research in superheavy synthesis and quantum materials.
The Ptable as a Predictive Tool in Material Innovation
Beyond classification, the Ptable drives innovation by enabling targeted element alloying and composite design. The discovery of high-temperature superconductors, such as yttrium barium copper oxide (YBCO), emerged from understanding copper oxide planes within Group 12. Similarly, lithium-ion batteries rely on precise lithium-intercalation chemistry derived directly from periodic group behavior—lithium’s low atomic mass and strong reducing power make it ideal for rechargeable storage.
“Every time we design a new material, we’re reading the periodic table backwards—using known trends to anticipate peak performance,” says Dr. Elena Morales, a materials scientist at the National Institute of Standards and Technology.
The table also illuminates environmental and health considerations. Cadmium’s toxicity and mercury’s bioaccumulation link back to their position in Period 6, Group 12 and 12, while lead’s stability in Group 14 reflects its long-lasting, problematic environmental persistence.
“Mapping risk requires knowing an element’s periodic personality,” Morales adds. “The Ptable guides safer, smarter material choices.”
Quantum Mechanics Redefined Through Periodic Structure
The modern Ptable’s architecture arises from quantum theory—specifically, electron shell filling and orbital hybridization. The Aufbau principle, Pauli exclusion, and Hund’s rule determine how electrons occupy 1s, 2s, 2p, and beyond, shaping atomic size, ionization energy, and chemical reactivity.
Subshell filling explains why atomic radii shrink across periods (increasing nuclear charge pulls electrons closer) yet expand down groups (additional shells offset higher effective nuclear charge). “The periodic table is quantum mechanics made visible,” states Dr. Mark Chen, a quantum chemist.
“Each element’s position reflects the unique dance of its electrons.” This quantum foundation underpins predictive modeling of novel compounds and exotic states of matter, from topological insulators to high-pressure superconductors.
Looking Forward: The Ptable and the Future of Chemistry
As researchers probe elements beyond the traditional periodic table—including synthetic superheavy nuclei and quantum dystionary states—the Ptable continues to adapt. Proposals for “super-table” layouts incorporate relativistic effects, critical for elements like oganesson (Og, Atom 118), where electron speeds near the nucleus alter chemical behavior predicted by standard models. Meanwhile, artificial intelligence and machine learning are accelerating element analysis, parsing Ptable data to identify patterns invisible to human scrutiny.
“The periodic table is no longer static,” says Prof. Liu Wei, a periodic systems theorist. “It’s evolving dynamically, fueled by quantum computing and deep data analysis.”
Whether guiding lab experiments, industrial R&D, or academic teaching, the Ptable remains the cornerstone of chemical literacy.
Its organized chaos reveals the fundamental logic behind all matter, turning elemental chaos into coherent order. As humanity ventures into greener chemistry, quantum materials, and space-based manufacturing, the Ptable persists not merely as a chart—but as a living blueprint of nature’s most basic building blocks.



Related Post

Remembering Putri Ariani: A Life Set Ablaze in the Dawn of a New Era

Unlock Virtua’s Power: Solo Leveling Game Wiki’s Ultimate Guide to Ascending Through Kiln of Rank

Lamar Jackson’s 40 Yard Dash: Did He Run at the NFL Combine?

How Hero Company Baums Neukunftmit Der Perfekte Newcomer: The Seven-Step Journey to Exceptional Onboarding

