Decoding Calcium: The Lewis Dot Structure That Reveals Its Atomic Identity
Decoding Calcium: The Lewis Dot Structure That Reveals Its Atomic Identity
Calcium, a pivotal element in biochemistry and materials science, defies the veil of mystery surrounding many alkaline earth metals through its elegant Lewis dot structure. With atomic number 20, calcium’s electron configuration and valence arrangement not only define its chemical behavior but also underpin its role in cellular signaling, bone formation, and industrial applications. Understanding calcium’s atomic spirit—how its electrons are shared and positioned—offers critical insight into its reactivity and utility.
At the core of calcium’s chemical identity lies its Lewis dot structure, a graphical representation that maps valence electrons around the atomic nucleus. Calcium, located in Group 2 of the periodic table, possesses two valence electrons—confirmed by its electron configuration: 1s² 2s² 2p⁶ 3s². The dot structure places these two electrons either opposite each other on the 3rd shell or paired, typically visualized as Ca··· or simplified as Ca·—representing its intent to achieve stability through two electron loss, forming a +2 charge.
Visualizing calcium’s Lewis structure reveals a clear pattern: the 3s orbital holds two dots, symbolizing the electron pair readily released in ionic bonding.
When calcium cedes these electrons—as it does in reactions with water or acids—its noble-gas-like configuration (mimicking xenon) emerges, marking a fundamental shift from reactive metal to stable, biologically vital cation.
Interestingly, calcium’s dot structure does not show unpaired electrons; instead, the 3s orbital’s two dots are paired, reflecting the element’s tendency to lose two electrons fully rather than exhibit variability in oxidation states—unlike transition metals. This electron behavior aligns with calcium’s classification as a Group 2 metal and explains its uniform +2 oxidation state across common compounds. The dot arrangement underscores its monovalence: one predictable charge, two stable interactions.
- Its filled d-orbitals in lower energy states contribute to structural rigidity, making calcium ideal for bone and teeth scaffolding. - The absence of unpaired electrons ensures predictable reactivity, critical in pharmaceuticals and soil health applications.
Delving deeper into the structural geometry, calcium’s Lewis structure reveals no lone pairs beyond the two valence dots, resulting in a bent or linear orientation depending on bonding partners.
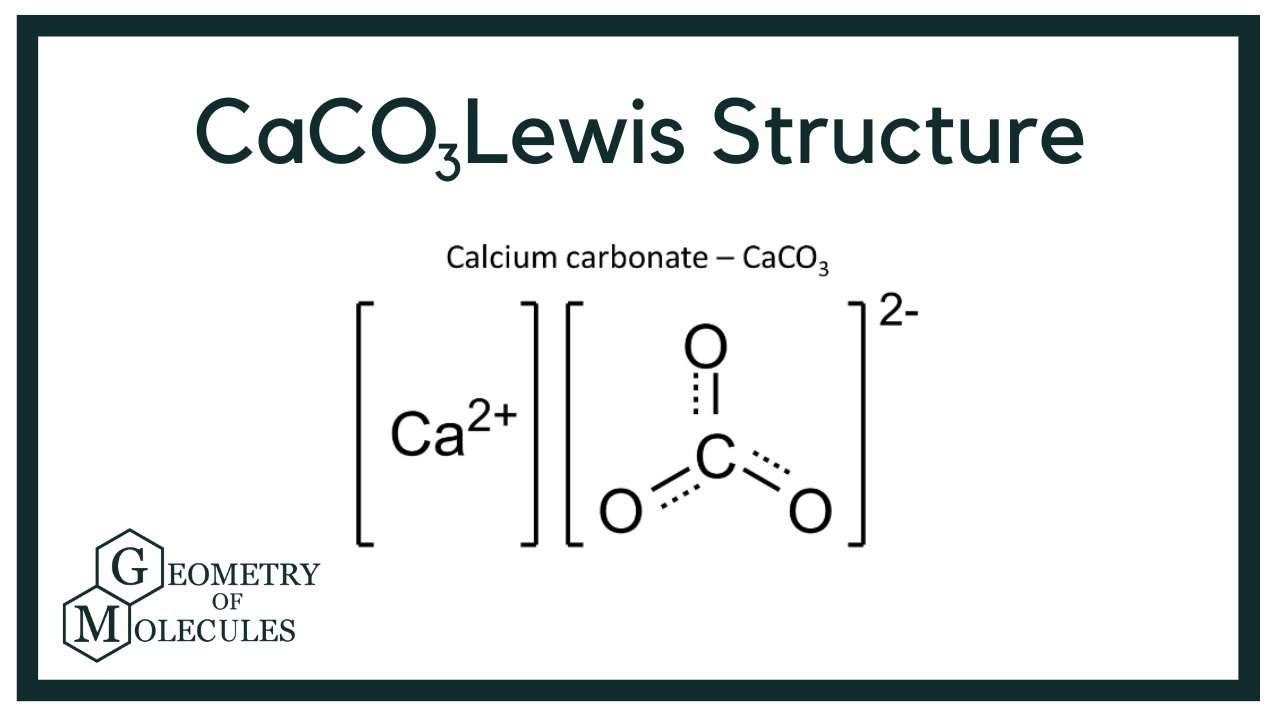
When ionically bound—such as in oxides (CaO) or carbonates (CaCO₃)—calcium adopts a hexacoordinated environment, where oxygen atoms arrange symmetrically around the cation in octahedral geometry. This coordination reinforces the +2 charge stability and ensures structural integrity in minerals and biological tissues.
In compounds like calcium carbonate (CaCO₃), the Lewis structure shows oxygen atoms using standard two-electron lone pairs, complementing calcium’s two donated electrons to form a neutral, stable ionic lattice. This combination of coordination and charge balance enables calcium’s widespread presence in biological systems, from neurotransmitter release to nerve conduction.
Beyond bonding, calcium’s electron arrangement influences its physical properties: high thermal conductivity, malleability, and solid-state stability at room temperature.
These characteristics stem from metallic bonding in elemental form and ionic bonding in compounds—both rooted in the predictability revealed by Lewis dot representations. Calcium’s behavior in lab settings, including its vigorous reaction with water (producing Ca(OH)₂ and hydrogen gas), directly follows from this electron logic—ionizable via electron transfer, not complex orbital mixing.
In scientific and industrial contexts, the Lewis dot structure of calcium is more than a pedagogical tool—it is a foundational framework for predicting reactivity, designing new materials, and optimizing biological and chemical processes. From cement production to cellular signaling, calcium’s behavior is dictated by the simple elegance of two valence electrons willing to link and stabilize.
This atomic insight ensures researchers, students, and engineers alike interpret calcium’s role with precision and confidence, reinforcing its status as a cornerstone of inorganic chemistry.



Related Post

Nadia Nakai: The Maverick Behind Crtry’s Ascent in the Evolution of Crypto Infrastructure

How Many Novels Has Stephen King Written? A Definitive Count and Literary Legacy
Basket Random Unblocked: The Ultimate Unfiltered Gaming Gateway

How Long Ago Was 2017: Riding the Tech Wave of a Decisive Year

