Decode the Invisible: How Nitrogen’s Lewis Dot Structure Illuminates Key Chemical Behavior
Decode the Invisible: How Nitrogen’s Lewis Dot Structure Illuminates Key Chemical Behavior
Nitrogen, the cornerstone of life, plays a surprisingly complex role in chemistry—often operating behind the scenes in molecular interactions. Understanding its electron arrangement through Nitrogen’s Lewis Dot Structure reveals not just atomic details, but profound insights into reactivity, bonding, and role across biological and industrial systems. By mapping valence electrons with precision, Lewis structures transform abstract quantity into visual truth, unlocking the logic behind nitrogen’s ubiquitous yet enigmatic chemistry.
The Pixelated Blueprint: Building the Nitrogen Lewis Dot Structure
At its core, a Lewis Dot Structure simplifies the electron world—those fleeting particles that dictate chemical bonds and molecular identity.
For nitrogen, a group 15 element with five valence electrons, the structure follows a clear logic: nitrogen forms simple covalent bonds by sharing electrons, always leaving an unpaired electron, consistent with its nitrogen atom’s electronic configuration: 1s² 2s² 2p³. This configuration directly informs its Lewis representation.
The standard Lewis Dot Structure for nitrogen consists of seven dots—six representing outer-shell electrons and one symbolizing the unpaired electron. These dots are positioned around or near nitrogen’s symbol to show bond formation:
- Two single bonds displaying two dots each attached to nitrogen, using two of the five valence electrons.
- The remaining three electrons manifest as a lone pair in a perpendicular pair, completing nitrogen’s octet-like stability (even though nitrogen lacks a full octet, it frequently achieves stability through bonding).
- A third dot may appear offset (often top or bottom) to visually balance symmetry and reflect the molecule’s inherent directionality.
This minimalist yet rich diagram serves as more than a teaching tool—it reveals nitrogen’s electron-sharing strategy and hints at molecular geometry, reactivity, and stability.
Electron Dynamics: The Role of the Unpaired Electron
The most pivotal feature in nitrogen’s Lewis structure is its lone unpaired electron.
Unlike highly paired configurations, this transient electron defines nitrogen’s behavior as both a weak nucleophile and a potential radical under certain conditions. In ammonia (NH₃), nitrogen’s lone pair enables it to accept protons, driving key reactions in biological systems, while in nitrogen gas (N₂), the triple bond locks the unpaired electrons into a resilient lattice, explaining nitrogen’s famously inert nature at room temperature.
Quantifying nitrogen’s reactivity through this electron lens clarifies phenomena like ammonia’s role in the Haber-Bosch process and nitric oxide’s signaling in cellular communications. As chemist James M.
Robinson notes, “The ability of nitrogen to stabilize through partial electron sharing—visible only in its Lewis structure—is nature’s masterclass in controlled reactivity.”
Bonding Patterns and Molecular Architecture
While nitrogen typically forms three covalent bonds—each with a shared pair—its Lewis structure reveals subtler bonding nuances. The nitrogen triple bond in N₂, visualized as one large bond represented by three pairs of dots alongside a double bond line (though not explicitly drawn in Lewis notation), underscores the strength of triple-bond stabilization. This bond holds together the diatomic molecule’s exceptional thermal and chemical inertness, a trait fundamental to atmospheric chemistry and industrial inerting processes.
In contrast, ammonia (NH₃) displays tetrahedral geometry with nitrogen bonded to three hydrogen atoms and retaining its lone pair.
The lone pair repels bonding orbitals, compressing H-N-H bond angles to 107°, slightly less than the ideal tetrahedral 109.5°—a classic example of valence shell electron pair repulsion (VSEPR) theory visualized through Lewis framework principles. This distortion directly influences hydrogen bonding, solubility, and ammonia’s biological activity.
Nitrogen’s Ladysquare: Reactivity and Environmental Impact
Understanding nitrogen’s Lewis structure is key to decoding its varied roles—from vital nutrient in fertilizers to a driver of pollution. In nitrogen fixation, enzymes leverage nitrogen’s capacity to form transient bonds with transition metals, reducing the high-energy triple bond to deliver bioavailable ammonium (NH₄⁺).
Yet, in industrial Haber-Bosch synthesis, controlled breaking and reforming of N₂ bonds demand precise electron management, balancing energy costs with output efficiency.
Equally notable is nitrogen’s environmental duality. While inert N₂ dominates Earth’s atmosphere (78%), reactive intermediates—like nitric oxide (NO) and nitrogen dioxide (NO₂)—carry out critical redox chemistry. NO, formed in combustion via nitrogen’s lone electrons accepting oxygen’s orbitals, signals cellular signaling and ozone cycling, yet contributes to smog and acid rain.
These reactive nitrogen species are visible in the Lewis structure only when accounting for electron mobility and transient pairing.
Visual Precision: Why the Nitrogen Lewis Dot Structure Matters Beyond the Classroom
In educational settings, the Nitrogen Lewis Dot Structure excels as a conceptual bridge—making abstract electron concepts tangible. But its importance extends into spectroscopy, computational chemistry, and molecular design. Accurate Lewis models inform spectroscopic transitions, guide molecular docking simulations, and enable chemists to predict reactivity before synthesis.
As a visual anchor, it streamlines complex electronic systems into strategic insights, empowering both students and researchers to decode nitrogen’s silent but colossal influence on matter, life, and technology.
From guiding biological catalysts to shaping industrial innovation, the Nitrogen Lewis Dot Structure remains more than a symbolic sketch—it is a foundational lens through which the invisible choreography of electrons becomes understood, predicted, and harnessed. In the quiet arrangement of dots and lines around nitrogen, chemistry breathes clarity, revealing the invisible forces that bind the world together.




Related Post

The Ice Age Sid That Became a Symbol of Resilience and Evolution

Unlock Endless Cinema: Discover Free Movies Anytime on Watchfreemovies Com
Pseihimanshuse Traders: Unlocking Ajmer’s Trading Gold via Timeless Local Wisdom
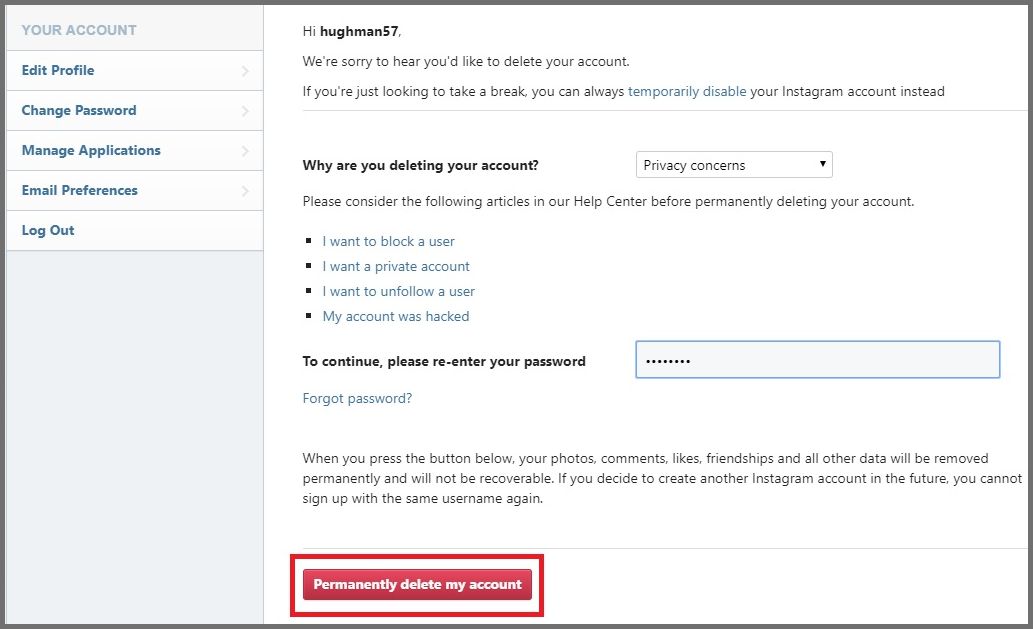
Deactivate Instagram Account: A Quick, Step-by-Step Guide to Remove Permanently

