Constitutional Isomers: The Molecular Double Thinkers Shaping Chemistry and Innovation
Constitutional Isomers: The Molecular Double Thinkers Shaping Chemistry and Innovation
Beneath the surface of every stable molecule lies a world of molecular deception—constitutional isomers, chemical twins that share the same formula but diverge in function, shape, and behavior. These structural variants challenge the assumption that molecular identity is written solely in atomic composition, revealing instead how subtle rearrangements can redefine reactivity, stability, and application. From pharmaceuticals to natural compounds, constitutional isomers illustrate the nuanced complexity underpinning chemistry’s promise—and peril.
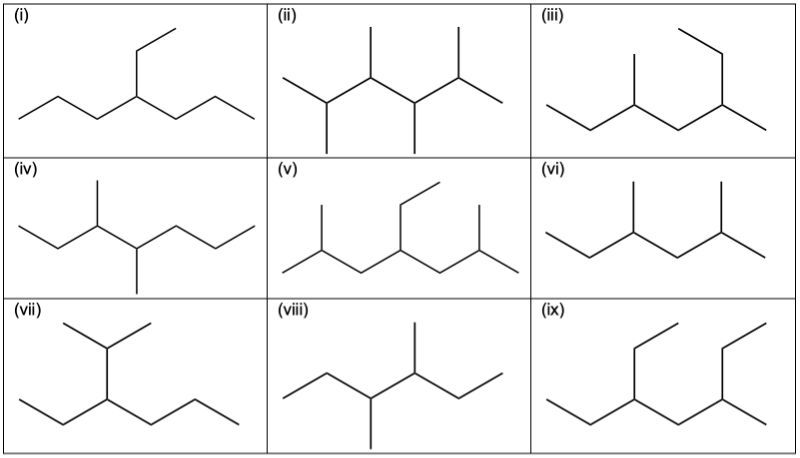
At their core, constitutional isomers are compounds with identical molecular formulas but distinct connectivity of atoms. This means every atom is present exactly once, but the architecture governing their bonds differs completely. For example, butane (C₄H₁₀) and iso-butane (C₄H₁₀), though sharing four carbons and ten hydrogens, branch into entirely different geometric forms—one linear, the other branched—yielding measurable differences in boiling points, combustion efficiency, and chemical reactivity.
This very principle defines a hidden layer of molecular diversity that drives both natural processes and human innovation.
The Structural Divide: Types and Tactics of Molecular Reconfiguration
Constitutional isomers arise through several distinct structural arrangements, each with unique implications. Recognizing these types clarifies how isomerism shapes chemistry at both macro and micro levels.Chain Isomers: The Linear vs.
Branched Path Chain isomers occur when carbon skeleton connectivity varies. A classic example lies in the C₄H₁₀ family: butane (straight chain) and iso-butane (branched)—though both follow the same atomic count, their architecture alters physical properties. Butane’s linear framework maximizes surface contact, lowering its boiling point compared to iso-butane, where compact branching reduces intermolecular forces.
This difference influences fuel efficiency and gas storage, with iso-butane favored increasingly in modern liquefied petroleum gas (LPG) blends for improved volatility and heat output. “Chain branching is not just geometric—it’s a control knob for material performance,” explains Dr. Elena Martinez, organic chemist at MIT.
“In fuels, slight changes in carbon skeleton geometry can dramatically shift performance metrics.”
Position Isomers: Bond Placement Matters
Position isomers differ in where atoms—especially functional groups—are positioned along a carbon chain. Acetone (CH₃COCH₃) and methyl ethyl ketone (CH₃CH₂COCH₃) illustrate this: though both ketones, their carbon group placement alters polarity and solvent behavior. Such precision enables tailored use in chemical synthesis.Acetone dissolves plastics and resins effectively, while the position variant excels in organic reactions requiring specific orientation. “Isomer-specific reactivity determines how molecules engage in chemical dance,” notes Professor Rajiv Nair, expert in stereochemistry. “Position shifts can unlock pathways unavailable in their linear counterparts.”
Group Isomers: Functional Group Relocations
Group isomers involve repositioning of entire functional groups across carbon skeletons.A notable instance is tariffic acid (HOOC-CH₂-CH₂-COOH) and methyl trifluoroacetate (CH₃FO₂-CH₂-CH₂-COOH). Despite sharing the C₄H₆O₄ formula, their functional group placement drastically affects polarity, solubility, and reactivity. Tariffic acid’s symmetric carboxyl groups enhance hydrogen bonding, boosting its role as a natural preservative, while methyl trifluoroacetate’s electron-withdrawing fluorine enhances reactivity in synthetic catalysts.
This functional rearrangement proves pivotal in drug design and industrial chemistry, where minute structural shifts determine biological impact and process efficiency.
The Synthesis and Detection Challenge
Creating constitutional isomers demands precise control over reaction conditions and molecular blueprinting. Synthetic routes often leverage catalytic isomerization, selective oxidation, or stepwise structural modification to steer bonds into desired configurations.Yet, their detection remains nuanced; standard spectroscopy may miss differences without advanced tools. Nuclear Magnetic Resonance (NMR) spectroscopy, for instance, distinguishes isomers via unique chemical shifts—imagine two faces of the same molecule, visible only through precise magnetic resonance analysis.
Mass spectrometry and X-ray crystallography further complement by mapping molecular weight and three-dimensional structure, ensuring accuracy in identifying isomers that differ by mere bond pathways but not static form.
Industrial Impact: Fuels, Pharmaceuticals, and Beyond
Constitutional isomers underpin critical industrial applications.In the fuel sector, iso-butane’s enhanced combustion properties improve engine efficiency and reduce emissions. Meanwhile, isoprene (C₅H₈)—a key isomer in natural rubber—drives synthetic polymer production, underpinning tire manufacturing and medical devices. In pharmaceuticals, isomerism can mean ethical and therapeutic divergence.
A well-known case: thalidomide’s tragic history traces to one isomer’s sedative properties and the other’s severe teratogenic effects—highlighting how structural precision safeguards human health. “Isomer awareness is now a cornerstone of green chemistry and precision engineering,” says Dr. Li Wei, a chemical safety researcher.
“Selecting the right isomer isn’t just about performance—it’s about responsibility.”
Navigating the Isomer Landscape: Challenges and Future Horizons
Identifying and harnessing constitutional isomers remains a frontier in chemical science. Computational modeling now predicts isomer stability, accelerating discovery cycles. Machine learning algorithms parse vast chemical databases, flagging promising candidates for synthesis and testing—reducing trial-and-error and environmental impact.Yet, complexities persist. As molecules grow longer, isomeric combinations multiply exponentially, challenging conventional analysis. Moreover, subtle isomer differences sometimes escape routine detection, requiring ever-advanced instrumentation to maintain safety and efficacy.
The rise of isomer-aware chemistry signals a paradigm shift: nature’s molecular grammar, once overlooked, now shapes innovation across energy, healthcare, and materials. By mastering structural nuance, chemists unlock smarter, safer, and more sustainable solutions—each bend in the bond line a step toward a higher-functioning world.
Constitutional isomers, often invisible to casual observation, stand as testament to chemistry’s depth.
Their existence challenges assumptions, expands possibilities, and demands precision beyond mere formulas. As science continues to decode their behavior, these molecular twins will guide the next generation of discovery—proving that identity lies not only in what atoms carry, but how they are connected.


Related Post
Unmasking Darkness: The Unforgettable Ensemble of Coven American Horror Cast

Arlington Toyota Palatine: Honest Reviews & Insights — What Drivers Really Think

The Fall of Nikki Catsouras: How a Tragic Moment Shocked Florida and Sparked a Media Lightning Bolt

Unbanned G: The Resilient Force Transforming Digital Rights and Creative Expression