Atomic Mass of Aluminum: The Key to Understanding One of Earth’s Most Versatile Metals
Atomic Mass of Aluminum: The Key to Understanding One of Earth’s Most Versatile Metals
Aluminum, the eighth most abundant element in Earth’s crust, plays an indispensable role in modern industry, transportation, and technology—from aircraft frames and skyscraper skeletons to everyday packaging and electronics. Yet, behind its lightweight grace lies a precise atomic foundation: its atomic mass, approximately 26.98 u (atomic mass units), is not just a number—it’s the cornerstone of its physical and chemical behavior. Understanding the atomic mass of aluminum reveals not only its identity but also its utility, from guiding alloy development to enabling accurate scientific modeling.
At the heart of materials science, the atomic weight of an element determines how atoms interact in compounds, influencing density, strength, thermal conductivity, and electrical behavior—parameters critical to thousands of applications. Aluminum’s atomic mass of 26.98 u arises from the weighted average of its naturally occurring isotopes, primarily aluminum-27, which constitutes over 99% of natural aluminum. This value reflects a balance between stability and abundance, with trace amounts of aluminum-26 and aluminum-25 contributing to the final figure.
Unlike synthetic elements with artificial isotopic compositions, aluminum’s atomic mass is dictated by nature’s equilibrium, offering scientists a reliable benchmark.
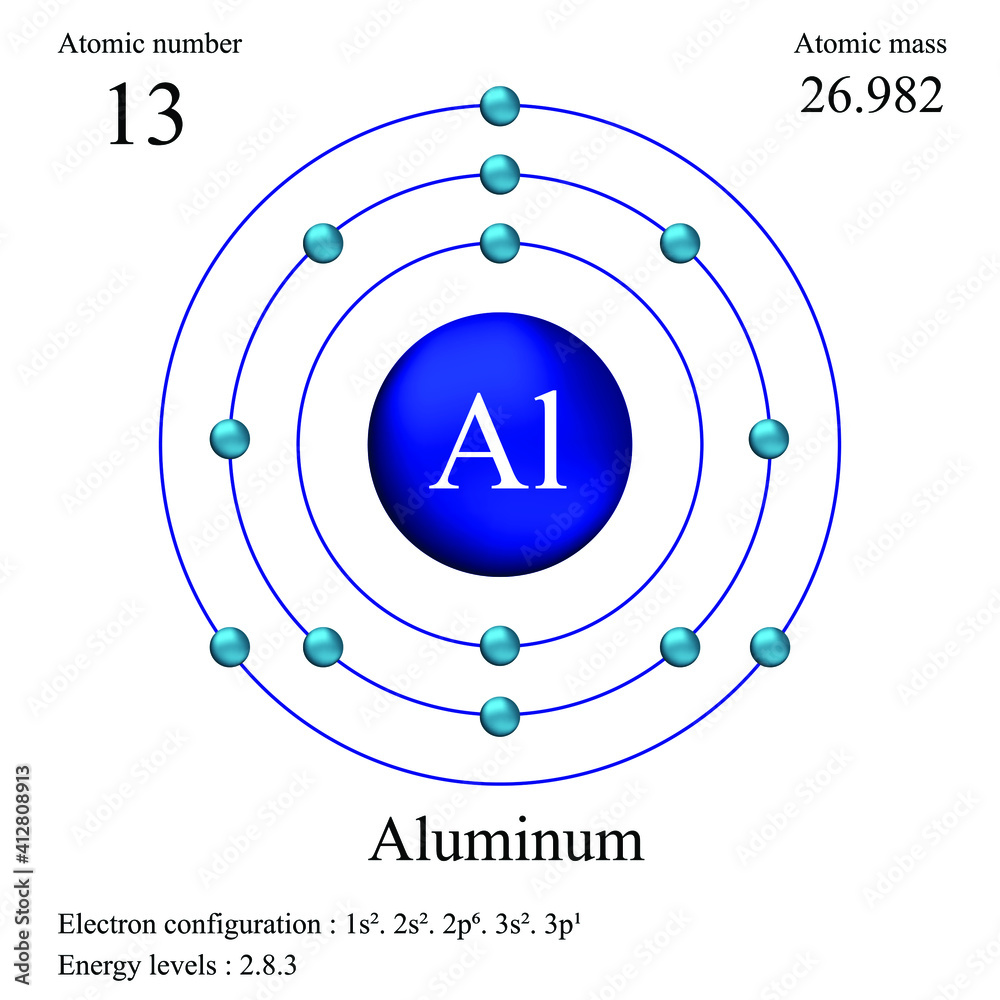
Isotopic Composition and Natural Abundance
The atomic mass of aluminum is derived from its isotopic distribution. Natural aluminum consists of 26 atomic mass units (u) weighted by the percentages of its stable isotopes: - Aluminum-27 (²⁷Al) makes up 96.48% of natural samples, - Aluminum-26 (²⁶Al) accounts for 0.27%, - Aluminum-25 (²⁵Al) contributes less than 0.01%. Using precise isotope abundance data and atomic masses—26.9854 u for ²⁷Al, 25.9868 u for ²⁵Al, and 26.9839 u for ²⁶Al—the weighted average yields: (0.9648 × 26.9854) + (0.0027 × 25.9868) + (0.0001 × 26.9839) ≈ 26.98 u.This value underscores aluminum’s stability and Earthly prevalence, aspects that engineers exploit to design reliable, high-performance materials. "With ²⁷Al dominating nearly all natural samples, aluminum’s atomic mass reflects both its cosmic abundance and robust isotopic harmony," notes Dr. Elena Torres, materials physicist at the Institute of Advanced Metals Research.
"This uniformity ensures predictable behavior in metallurgical processes."
Unlike elements with high isotopic variance—such as lithium, where isotopic distribution ranges widely—aluminum’s isotopic profile is exceptionally narrow. This uniformity simpl



Related Post

Unveiling the Timeless Voice Behind Tangled: The Cast That Brought Rapunzel to Life

Pikeville Restaurants Ky: Where Tradition Meets Innovation in Appalachian Cuisine

The Way Of Kings Audiobook Free: Access Epic Fantasy Like Never Before

Who Stands Behind Matt Czuchry? Unveiling the Wife Shaping the Actor’s Life in 2024

